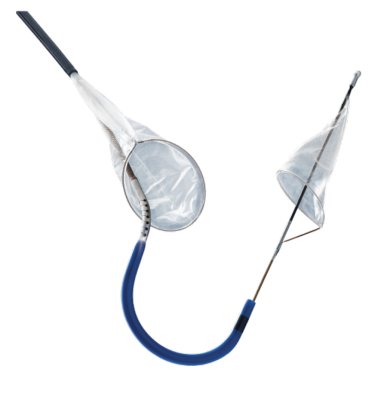
September 17, 2022 — Boston Scientific Corporation has announced results from the PROTECTED TAVR clinical trial evaluating the SENTINEL Cerebral Protection System, which is designed to capture and remove embolic debris stemming from transcatheter aortic valve replacement (TAVR) before it can reach the brain and potentially cause a stroke. Outcomes were presented during a late-breaking clinical science session at the 34th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Boston, and will be published in the New England Journal of Medicine.
This randomized trial evaluated periprocedural stroke reduction and neurologic outcomes in patients with aortic stenosis treated with either the SENTINEL device to provide cerebral embolic protection (CEP) during TAVR or TAVR alone. The primary endpoint was not met, as the data demonstrated a non-significant trend towards a lower rate of stroke in patients treated with the SENTINEL device, representing a 21% relative risk reduction in all stroke through 72 hours or time of hospital discharge (2.3% with TAVR and CEP vs. 2.9% with TAVR only, P=0.30). Importantly, a secondary analysis demonstrated a statistically significant 60% relative risk reduction in disabling stroke through 72 hours or time of hospital discharge in patients treated with the SENTINEL device (0.5% with TAVR and CEP vs. 1.3% with TAVR only, P=0.02).
"Data from the PROTECTED TAVR trial provide the physician community with evidence that the device plays an important role in reducing disabling strokes across patient types in those undergoing TAVR," said Dr. Samir Kapadia, chairman of the Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Sydell and Arnold Miller Family Heart, Vascular & Thoracic Institute, Cleveland Clinic. "We also found that the rate of vascular complications in this trial was very low, whether or not the device was used, highlighting the safety of this technology in TAVR procedures."
While outcomes with TAVR for the treatment of aortic stenosis have been shown to be comparable to surgery, stroke remains an important and feared complication of the procedure. Representing the largest randomized TAVR trial to date, the study enrolled 3,000 patients spanning more than 50 global sites and all surgical risk levels, with all patients receiving a neurological exam before and after the procedure. Subgroup analyses demonstrated that the reduction in disabling stroke with the SENTINEL device was consistent across patient subgroups, including age, gender, operative risk, valve type and history of cardiovascular disease.
"Considering strokes are unpredictable, can occur regardless of an individual's clinical background and often take a great toll on a patient's quality-of-life and financial stability, we believe the data appear to demonstrate a consistent effect from CEP technology across all patient populations in the trial – supporting the use of the SENTINEL device as an effective therapy to reduce the risk of the most debilitating form of stroke for patients undergoing TAVR," said Dr. Ian Meredith, global chief medical officer, Boston Scientific. "We look forward to additional data on this technology such as from the currently enrolling PROTECT TAVI trial in the United Kingdom, which will similarly evaluate TAVR-related stroke reduction using the SENTINEL device."
Previous clinical trials involving more than 3,500 patients have demonstrated that the SENTINEL device is safe and effective, including capture and removal of cerebral embolic debris in 99% of TAVR cases.1 To date, more than 75,000 patients worldwide have been protected with this technology.
For more information: www.bostonscientific.com/sentinel


 October 31, 2025
October 31, 2025 









