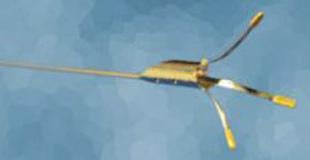
December 6, 2010 – Cordis Corp. has entered into a distribution agreement with Ostial Solutions for the worldwide distribution of the Ostial Pro Stent Positioning System.
The Ostial Pro is the only aorto-ostial stent positioning system for coronary and peripheral applications. It is intended to optimize the treatment of patients who require a stent implantation for treatment for aorto-ostial blockages.
Aorto-ostial blockages occur at the origin of blood vessels arising from the aorta. This occurs in the right and left main coronary arteries, in coronary bypass grafts and in the renal arteries. These blockages comprise approximately 95 percent of all renal stent interventions and 8 percent of coronary stent interventions.
It is critical for the end of the stent to align precisely with the origin of the blood vessel in these cases. Using two-dimensional X-ray imaging and angiography is challenging, time-consuming and can lead to increased cost and length of procedures, as well as increased dye and radiation exposure. Imprecise positioning often requires the use of additional stents to treat the lesion and may increase risks of renarrowing.
“Ostial Pro is a simple solution to a principal challenge of aorto-ostial stent positioning by providing visual and tactile identification of the true ostium,” said Campbell Rogers, M.D., chief scientific officer and global head of research and development, Cordis. “The Ostial Pro facilitates precise stent implantation of aorto-ostial stents by identifying the true ostium. This can help make precise stent placement easier, safer and more predictable. This technology may reduce the need for additional stents and may reduce the risk of having to perform another intervention for restenosis. Based on these benefits, this simple device should become a standard element of aorto-ostial stenting.”
The stent is FDA-cleared and is currently being sold only in the United States by Ostial Solutions. Cordis plans to assume worldwide distribution in the first half of 2011.
For more information: www.cordis.com, www.ostialsolutions.net


 November 24, 2025
November 24, 2025 









