May 20, 2010 – St. Jude Medical Inc. announced yesterday it is purchasing LightLab Imaging Inc. for $90 million. Lightlab makes optical coherence tomography (OCT) intravascular imaging systems. Earlier this month its OCT system became the first cleared by the U.S. Food and Drug Administration (FDA).
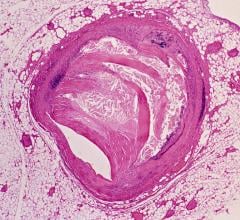
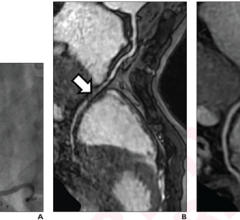
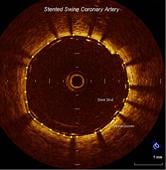
The high-resolution diagnostic coronary imaging technology aids physicians in the treatment of cardiovascular disease. OCT has been shown to provide image resolution 10 times greater than intravascular ultrasound (IVUS) imaging systems and 20 times faster image capture.
During the second half of 2010, St. Jude Medical expects the OCT platform to contribute an additional $20 million in revenue to its cardiovascular business. The OCT market is expected to grow at a double-digit compounded annual rate over the next five years and is expected to capture IVUS market share as well as expand the market for coronary imaging. The IVUS market is estimated to be $500 million for 2010, and is growing 10 to 15 percent annually. OCT coronary imaging is expected to grow at an even faster rate within this market.
OCT combined with FFR
Upon closing, St. Jude Medical will be the first company to offer a portfolio that includes both OCT and fractional flow reserve (FFR) technology. No other OCT systems are currently available for coronary imaging. This leading combination will provide physicians with comprehensive lesion assessment information, ranging from the anatomical images of OCT to the physiological data of FFR. St. Jude Medical acquired its FFR measurement technology platform through the acquisition of Radi Medical Systems AB in 2008. The LightLab business will become part of the St. Jude Medical Cardiovascular Division.
Lightlab’s OCT System
The FDA cleared LightLab’s C7-XR OCT imaging system and companion C7 Dragonfly imaging catheter is comprised of a console used in the cath lab and the catheter inserted into the vessel. This allows the clinician to readily see and measure important vessel characteristics otherwise invisible or difficult to observe with IVUS or angiography.
This next generation OCT system eliminates the need for temporary vessel occlusion that was required by the earlier generation OCT systems.
LightLab now has products approved in 40 countries, including the only OCT system cleared for use by regulatory bodies in the U.S., Europe and Japan.
Purchase details
The transaction is expected to close by the end of the second quarter 2010 and is subject to customary closing conditions and regulatory approvals. Except for a nonrecurring charge to be recorded at the time of closing, this acquisition does not change St. Jude Medical’s outlook for 2010 consolidated earnings per share.
In connection with the transaction, Bank of America Merrill Lynch is acting as financial advisor and Gibson, Dunn & Crutcher LLP as legal advisor to St. Jude Medical. Brown Brothers Harriman is acting as financial advisor to LightLab and Choate, Hall & Stewart LLP as legal counsel. Kitahama Partners, LLC is acting as legal counsel to Goodman Co. Ltd., the current owner of Lightlab.
For more information: www.lightlabimaging.com


 October 24, 2025
October 24, 2025