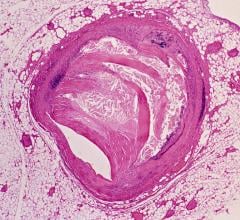
February 15, 2008 - Siemens received FDA 510(k) clearance for Artis zeego, a robotic-assisted positioning capability for interventions in both radiology and cardiology, as well as the developing OR environment, designed to offer greater flexibility of movement and image acquisition.
Rotational angiography delivers large-volume syngo DynaCT to visualize the entire abdomen or the entire thoracic spine showing the complete region of interest. Ergonomically designed working positions are available due to the system’s isocenter, in order to increase operator comfort during long and complex procedures. Parking positions maximize the use of room space during patient transfers. When the system is not in use it can be suited for the OR or hybrid environment.
Combined with the existing 2D and 3D features and applications common across the entire Artis zee family, the Artis zeego is poised to deliver enhancements in imaging and workflow.
For more information: www.usa.siemens.com/medical


 October 24, 2025
October 24, 2025