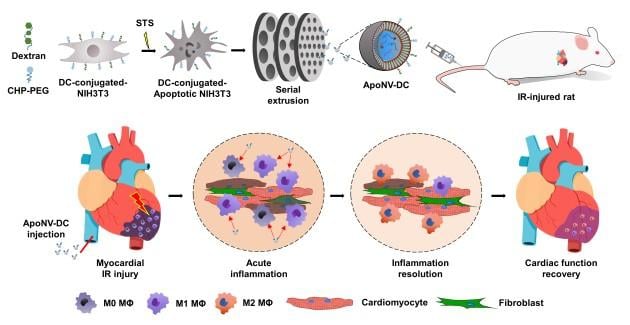
Schematic illustration of treatment of myocardial ischemia-reperfusion (IR) injury with the targeted delivery of ApoNV-DCs. Image courtesy of Korea Institute of Science and Technology
September 14, 2023 — Myocardial infarction, the number one cause of sudden death in adults and the number two cause of death in Korea, is a deadly disease with an initial mortality rate of 30%, and about 5-10% of patients die even if they are transported to a medical center for treatment. The number of myocardial infarction patients in Korea has been increasing steeply, from 99,647 in 2017 to 126,342 in 2021, an increase of 26.8% in five years. Until now, drug administration, percutaneous angioplasty, and arterial bypass surgery have been known as treatments, but they are difficult to apply to severe cases that do not respond to them.
Dr. Yoon Ki Joung and Dr. Juro Lee of the Biomaterials Research Center at the Korea Institute of Science and Technology (KIST), together with Prof. Hun-Jun Park and Dr. Bong-Woo Park of the Catholic University of Korea College of Medicine, have developed a new treatment for myocardial infarction that uses nanovesicles derived from fibroblasts with induced apoptosis to modulate the immune response.
Myocardial infarction is an ischemic heart disease in which the coronary arteries, the blood vessels that supply blood to the heart, become narrowed or blocked, resulting in insufficient blood supply to the heart muscle, which causes nutrient and oxygen deficiency in the myocardium, leading to poor heart function. According to market research firm Technavio, the global myocardial infarction therapeutics market is expected to reach $2.02 billion by 2026, at a CAGR of 4.7%. In recent years, stem cell-derived nanovesicles, such as exosomes, have been used to treat myocardial infarction by modulating the inflammatory response, but stem cells are difficult to produce in large quantities, limiting their economic viability.
The research team identified the possibility of treating severe myocardial infarction by reducing the inflammatory response in the heart muscle through a nanomedicine based on apoptotic cells, which are cells that commit suicide due to biochemical changes in their cells. This response was achieved by attaching peptides specific to the site of ischemic myocardial infarction and substances specific to macrophage phagocytosis to the surface of fibroblasts. To this end, the team developed anti-inflammatory nanovesicles that can be delivered specifically to macrophages at the site of myocardial infarction.
In animal studies, we found that intravenously injected nanovesicles were effectively delivered to the myocardial infarction site in rats and were specifically recruited to macrophages. As a result, the left ventricular ejection fraction, an indicator of the contractile force of the left ventricle, increased by more than 1.5 times compared to the control group for 4 weeks. In addition, the effects of reducing inflammation and fibrosis, and increasing blood vessels preservation rate enhanced cardiomyocytes survival, which resulted in cardiac function improvement.
"This is the first study to use nanovesicles produced from apoptosis-induced cells to treat myocardial infarction, and it has the advantage of being able to mass-produce them because it uses other cells rather than stem cells," said Dr. Yoon Ki Joung of KIST. "In the future, we plan to conduct a research to verify the effectiveness and safety of the treatment, including clinical trials, through a collaborative research with Catholic University of Korea Medical School and bio companies."
For more information: https://eng.kist.re.kr/


 November 24, 2025
November 24, 2025 









