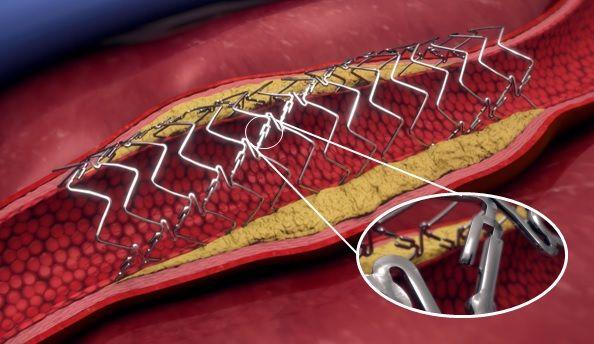
Elixir Medical has announced it has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its DynamX BTK System, a novel, adaptive implant for use in the treatment of narrowed or blocked vessels below-the-knee (BTK) in patients with chronic limb-threatening ischemia (CLTI). The company reports this broadens the use of the novel bioadaptor platform technology beyond the treatment of coronary artery disease. Image courtesy: Elixir Medical
March 27, 2024 — Elixir Medical has announced it has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its DynamX BTK System, a novel, adaptive implant for use in the treatment of narrowed or blocked vessels below-the-knee (BTK) in patients with chronic limb-threatening ischemia (CLTI).
The DynamX Bioadaptor platform is a medical technology designed to establish the new standard of care for vascular interventions, according to a written statement on the designation. It is the only metallic device designed to support the vessel during the healing phase, after which it unlocks and “uncages” the vessel while providing the essential dynamic support, to restore vessel function and maintain an open lumen.
Peripheral arterial disease (PAD) affects more than 200 million people worldwide.1,2 Its most severe form is CLTI, which represents an advanced stage of PAD with risk to both life and limb, and one-year estimates of mortality over 20% across a range of observational studies.3 Without revascularization, CLTI results in major amputation in a large number of patients.4, 5, 6
Because of its unique design and mechanism of action, the bioadaptor is a promising therapy in BTK revascularization, noted the statement from Elixir Medical, described as a developer of disruptive technologies to treat cardiovascular and peripheral disease. It further reported that the DynamX Bioadaptor has been shown to provide high acute lumen gain in the coronary vessels and maintain such gain over time, which is a common challenge in existing BTK therapies. The bioadaptor has also been shown to restore vessel motion and function, including positive adaptive remodeling, vessel pulsatility, improved vessel dynamic compliance, and an increase in blood flow volume.
"The Bioadaptor platform was developed to transform treatment of coronary and peripheral artery disease,” said Motasim Sirhan, CEO at Elixir Medical, based in Milpitas, CA. Sirhan added, “We appreciate the FDA recognition of our innovation for treating the CLTI population with BTK disease and its potential impact on the patients suffering from vascular disease.”
The FDA Breakthrough Device Designation accelerates the review process for novel technologies designed to address an unmet medical need. Devices that receive breakthrough designation must meet rigorous standards for device safety and effectiveness in order to be authorized for marketing.
DynamX Bioadaptor is the first implant technology designed to unlock the device, uncage the vessel, and restore and sustain normal vessel motion and function after PCI or BTK intervention by maintaining dynamic support of the diseased vessel after its uncaging. With this unique mechanism of action (MOA), it is designed to establish a new standard of care for vascular intervention.
The DynamX BTK System is not available for sale in the U.S.
More information: www.elixirmedical.com
References:
1 Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart. 2014;9(1):145-158 e121.
2 Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. 2013;382(9901):1329-1340.
3 Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, Conte MS and Murad MH. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642-51 e3.
4 Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S):3S-125S e140.
5 Rueda CA, Nehler MR, Perry DJ, McLafferty RB, Casserly IP, Hiatt WR, et al. Patterns of artery disease in 450 patients undergoing revascularization for critical limb ischemia: implications for clinical trial design. J Vasc Surg. 2008;47(5):995-999; discussion 999-1000.
6 Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62(6):1642-1651 e1643.


 January 15, 2026
January 15, 2026 









