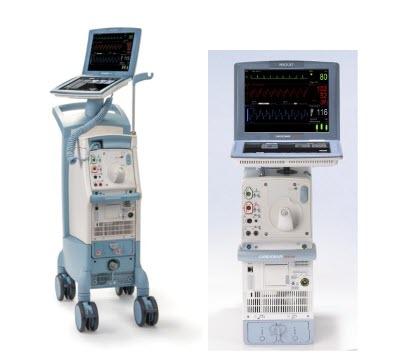
August 14, 2023 — The FDA has announced that Datascope/Maquet/Getinge is recalling the Cardiosave Hybrid and Rescue Intra-aortic Balloon Pumps (IABPs) because they may shutdown unexpectedly due to electrical failures in the Power Management Board and/or Solenoid Board (Power Source Path).
Using an affected pump may cause serious adverse health events, including unstable blood pressure, injury (for example: inadequate blood supply or a vital organ injury), and death.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death..
Recalled Product
Recalled Product:
- Cardiosave Hybrid Intra-Aortic Balloon Pump (IABP) and Cardiosave Rescue Intra-Aortic Balloon Pump (IABP)
Product Models:
Distribution Dates: March 6, 2012 to May 19, 2023
Devices Distributed in the U.S.: 4586
Date Initiated by Firm: June 5, 2023
Device Use
The Cardiosave Hybrid Intra-Aortic Balloon Pump (IABP) and the Cardiosave Rescue IABP are electromechanical systems used to inflate and deflate intra-aortic balloons. These systems provide temporary support to the left ventricle through counter pulsation. Once the balloon is positioned in the aorta, the pump is set to work in synchrony with the electrocardiogram or arterial pressure waveform to make the balloon inflate and deflate at the right time during the cardiac cycle.
Cardiosave Intra-Aortic Balloon Pumps are indicated for acute coronary syndrome, cardiac and non-cardiac surgery, or complications of heart failure in adults. They are used in health care facilities.
Reason for Recall
Datascope/Maquet/Getinge is recalling the Cardiosave Hybrid and Rescue Intra-aortic Balloon Pumps (IABPs) because they may shutdown unexpectedly due to electrical failures in the Power Management Board or Solenoid Board (Power Source Path).
Using an affected pump may cause serious adverse health events, including unstable blood pressure, injury (for example: inadequate blood supply or a vital organ injury), and death.
Datascope/Maquet/Getinge reported 26 complaints. There have been no reports of injuries, or death.
Who May be Affected
- People who receive circulatory support using a Cardiosave Hybrid or Rescue IABP
- Health care personnel providing care that includes the Cardiosave Hybrid or Rescue IABP
What to Do
On June 5, 2023, Datascope/Maquet/Getinge sent all affected customers an Important Medical Device Advisory.
The letter requested customers to:
- Ensure there is an alternative IABP available to continue therapy.
- Provide alternative hemodynamic support if there is no other means to continue counterpulsation therapy.
Contact Information
Customers with questions about this recall should contact their Datascope/Maquet/Getinge representative or call Datascope/Maquet/Getinge Technical Support at 1-888-943-8872, options 4, 2, 1, Monday through Friday, between the hours of 8:00 a.m. and 6:00 p.m. (Eastern Time).
Additional Resources:
Medical Device Recalls database
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.
Other recent FDA recalls:
FDA Issues Update on Abbott Trifecta Valves: Potential Risk of Early Structural Valve Deterioration
Abbott Recalls Amplatzer Steerable Delivery Sheath for Increased Risk of Air Embolism


 January 15, 2026
January 15, 2026 









