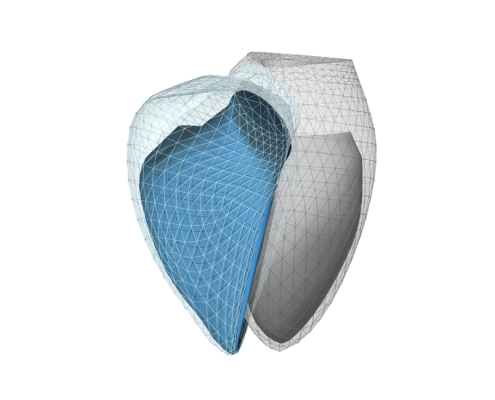
Software medical device developer Argus Cognitive has announced its ReVISION platform has received (510k) FDA clearance. ReVISION allows medical professionals to better diagnose and stratify right ventricular dysfunction in patients with cardiopulmonary diseases, according to the August 15 company statement. Image courtesy: Argis Cognitive.
August 15, 2023 — Argus Cognitive, Inc., a digital health company that develops software medical devices, announced that it has been granted U.S. Food and Drug Administration (FDA) 510k clearance for its 3D echocardiography-based product. ReVISION, as the software is called, allows medical professionals to better diagnose and comprehensively quantify global and segmental right ventricular function based on 3D echocardiographic models, according to a statement released by the company.
The company reports that while, currently, sophisticated solutions are available to model left ventricular morphology and function, right ventricular morphology and mechanics were traditionally less studied — in part due to its complex shape and contraction patterns. It notes that evidence is rapidly growing about the strong prognostic role of right ventricular performance in various cardiovascular diseases, yet no established tool was available for a detailed analysis until now.
With the input of 3D echocardiographic mesh models, ReVISION provides global and segmental functional parameters of the right ventricle, according the Argis Cognitive announcement. Beyond established markers of ventricular mechanics (longitudinal and circumferential strain), the software uniquely quantifies the longitudinal, radial and anteroposterior components of right ventricular wall motion and also provides segmental information. It is conveniently available from a web browser, requiring no local installation.
ReVISION won multiple innovation awards, including the American Society of Echocardiography’s ASE Echovation Challenge (2017). Argus Cognitive also reported that it is partnering with more than a dozen leading institutions worldwide and numerous papers have been published in reputable, peer-reviewed academic journals that either base their research on ReVISION or study ReVISION itself. According to literature data, this approach has further advantages in right ventricular function assessment as also highlighted in a recent Scientific Statement of the American College of Cardiology (ACC) published in its Journal of the American College of Cardiology, JACC, in May 2023. ReVISION reveals underlying mechanical changes in the right ventricle that other conventional metrics can miss.
Most cardiologists have access to 3D echo, but some have been reluctant to use it because of the limitations of available metrics, and because of the work involved in post-processing the 3D echo data, according to the company’s detailed announcement of its FDA approval. With ReVISION, they state, cardiologists can access much more detailed RV metrics easily, by simply uploading 3D DICOM files from 3D echo machines.
Argus Cognitive is a technology startup founded in 2016 by computer scientists, medical device developers, and clinicians. The company vision is to use state-of-the-art artificial intelligence technology to overcome the scalability problem seen in contemporary medicine, it noted, adding that treatment options and the amount of collected data have been increasing rapidly, yet clinicians' ability to process all this data cannot keep up with the demand. As such, the company notes that its non-invasive technology — software medical devices, regulated and delivered as Software as a Service (SaaS) — provide clinicians with decision support software that turns the data into comprehensive and quantitative information, and increase their throughput, thus helping them focus where their attention is most needed. The company’s product development team is based in Budapest, Hungary, and has offices in Hanover, NH.
More information: www.arguscognitive.com


 January 15, 2026
January 15, 2026 









