June 4, 2018 — The U.S. Food and Drug Administration said Medtronic initiated a Class 1 recall all 204,017 of its ...
Ventricular Assist Devices (VAD)
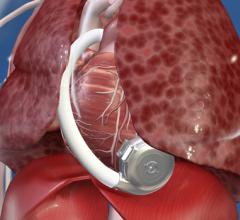
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
May 23, 2018 — Abbott has initiated a Class I recall of the HeartMate 3 Left Ventricular Assist System due to a ...
April 2, 2018 — Abiomed Inc. announced that it received U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA ...
David Lanfear M.D., FACC, head of advanced heart failure and cardiac transplantation, Henry Ford Hospital, Detroit ...
March 13, 2018 — At two years of follow-up, severely ill patients with advanced heart failure who received a novel heart ...
February 15, 2018 — Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre ...
February 14, 2018 — Abiomed Inc. announced it received an expanded U.S. Food and Drug Administration (FDA) pre-market ...
January 25, 2018 – According to the latest market study released by Technavio, the global circulatory support devices ...
December 11, 2017 — CorInnova Inc. recently announced it was awarded second prize in the “2017 InnoSTARS” life science ...
November 17, 2017 — Common practice, and recently published research, shows that the risk of complications with surgery ...
A discussion with William W. O’Neill, M.D., medical director, Center for Structural Heart Disease, Henry Ford Hospital ...
October 27, 2017 — CorInnova Inc. announced it has received notice of allowance of a seminal patent to protect its ...
October 26, 2017 — Medical professionals representing hospital systems from across the United States are expected to ...
October 4, 2017 — Medtronic received U.S. Food and Drug Administration (FDA) approval for its HeartWare HVAD System as a ...
September 28, 2017 — Abiomed Inc. recently received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) ...

 June 04, 2018
June 04, 2018