November 2, 2016 — St. Jude Medical Inc. announced the long-term data from RESPECT, a landmark trial, during a First ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
November 2, 2016 — Investigators recently unveiled clinical data from the independent BIO-RESORT study, representing the ...
November 2, 2016 — Medtronic plc recently announced new clinical data for the Harmony Transcatheter Pulmonary Valve (TPV ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
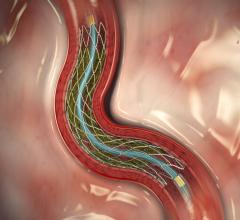
November 1, 2016 — Medtronic plc unveiled the first clinical outcomes of its novel Drug-Filled Stent (DFS) from the ...
November 1, 2016 — Edwards Lifesciences Corp. announced new five-year hemodynamic data from the PARTNER Trial ...
October 31, 2016 — Medtronic plc presented new positive data from two large registries aimed at evaluating 30-day ...
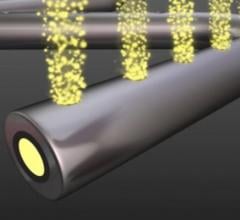
October 31, 2016 — Micell Technologies Inc. announced that it presented five-year clinical safety and efficacy results ...
October 31, 2016 — Optical coherence tomography (OCT) provides safe and improved guidance for patients undergoing ...

October 31, 2016 — A major international study has found that drug-eluting stents, a less-invasive alternative to bypass ...
October 28, 2016 — Mitralign will present data on its Trialign Tricuspid Repair system at the 2016 Transcatheter ...
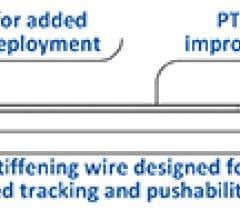
October 28, 2016 — Svelte Medical Systems Inc. announced this week it received CE Mark certification of the Direct ...
October 28, 2016 – Tryton Medical Inc., a primary developer of stents to treat coronary bifurcation lesions, and
...
October 27, 2016 — Corindus Vascular Robotics Inc. announced it received 510(k) clearance from the U.S. Food and Drug ...

The volume of information attendees receive at the annual Transcatheter Cardiovascular Therapeutics (TCT) interventional ...
October 27, 2016 — BioTrace Medical Inc. said it received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...


 November 02, 2016
November 02, 2016