January 25, 2013 — The American College of Cardiology (ACC) has released the late-breaking clinical trials that will be ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
January 22, 2013 — Cordis Corporation announced the presentation of the two-year STROLL clinical study results at the ...

January 16, 2013 — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver PTX drug ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 16, 2013 — Biotronik announced the first U.S. implant of their Pulsar-18 self expanding stent in the BIOFLEX-I ...
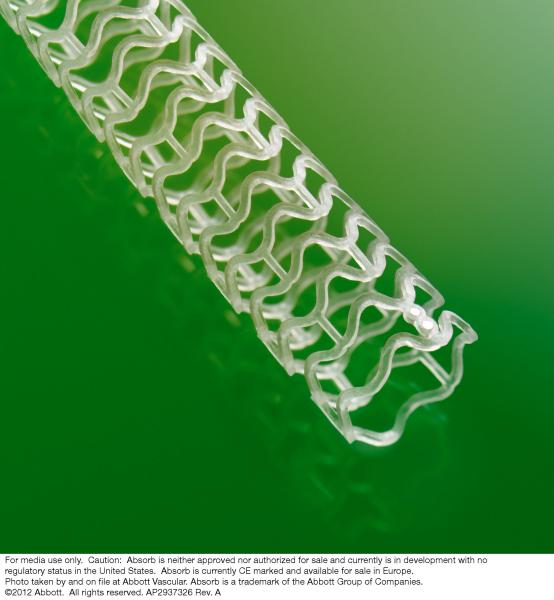
January 8, 2013 — Abbott announced the initiation of the ABSORB III clinical trial in patients in the United States. The ...
January 7, 2013 — Biosensors International has announced enrollment of the first patient in LEADERS FREE, a clinical ...
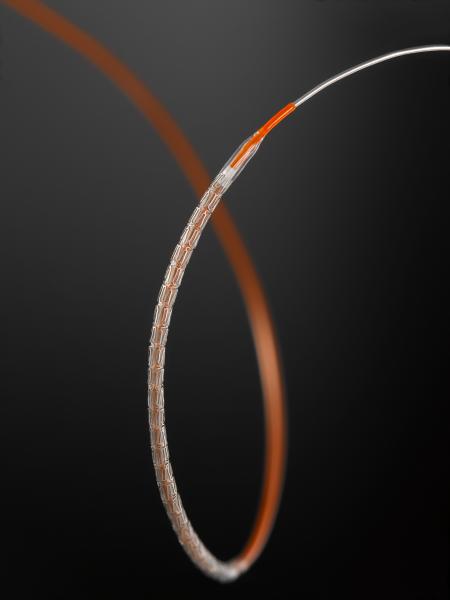
January 3, 2013 — Abbott announced that the Xience Xpedition everolimus-eluting coronary stent system received U.S. Food ...
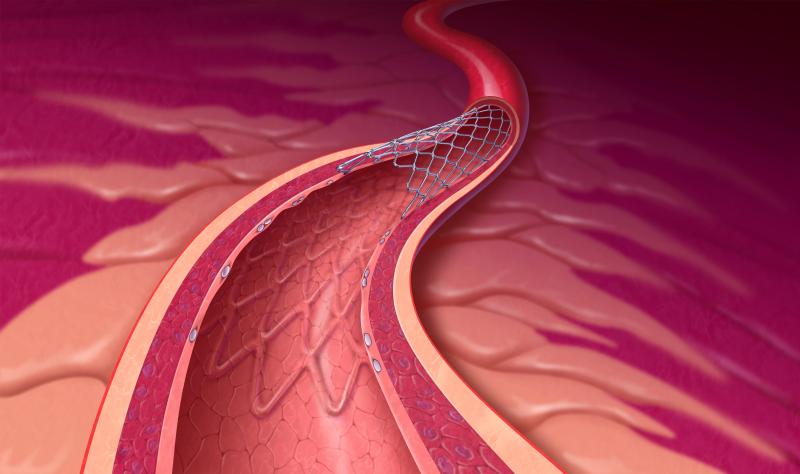
December 31, 2012 — When tests reveal that a patient’s heart artery is blocked and blood flow to the heart is restricted ...

December 28, 2012 — Resuscitation, cell regeneration, a new high blood pressure treatment and developments in devices ...
December 27, 2012 — Biotronik announced that the first implant has taken place in the BIOHELIX-I trial in the United ...
December 26, 2012 — Flexible Stenting Solutions Inc. (FSS) announced it gained CE mark in the European Union for its 6 ...
December 20, 2012 — The first patient has been enrolled in the Boston Scientific EVOLVE II clinical trial, which is ...
December 7, 2012 — More than half of patients treated for claudication or critical limb ischemia (CLI) with stenting of ...

December 7, 2012 — Tryton Medical Inc. announced the completion of enrollment in the TRYTON Pivotal U.S. Food and Drug ...

 January 25, 2013
January 25, 2013




