September 25, 2013 – The U.S. Food and Drug Administration (FDA) announced a final rule for the unique device ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
September 24, 2013 — W. L. Gore & Associates has enrolled the first patient in its Gore Carotid Stent clinical study for ...

September 23, 2013 — Cardiologists are increasingly accessing coronary arteries by way of the wrist rather than the ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 20, 2013 – OrbusNeich Medical Inc. and Boston Scientific Corp. have settled all stent-related litigation ...
September 20, 2013— The Mount Sinai Medical Center is participating in the nationwide Absorb III clinical trial testing ...
September 17, 2013 — In a significant milestone toward obtaining additional key regulatory approvals for the Synergy dru ...
September 9, 2013 — Cardiac stents to open blocked heart arteries and reduce chest pain have been used for decades ...
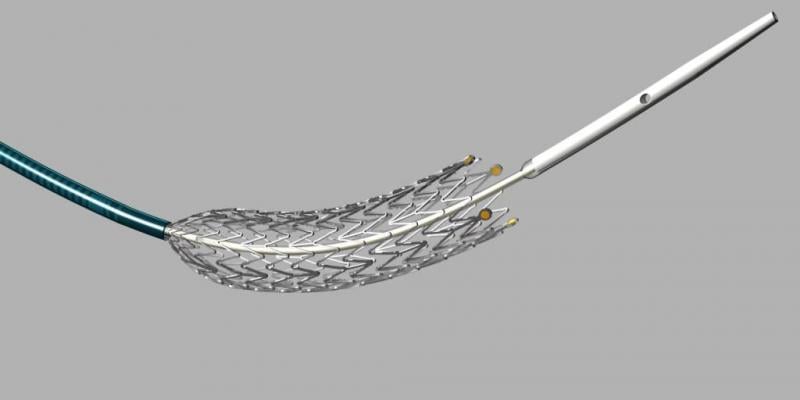
August 28, 2013 — Effective Oct. 1, Cook Medical’s Zilver PTX drug-eluting peripheral stent qualifies for new-technology ...
|
August 28, 2013 — According to Millennium Research Group (MRG), efforts to streamline the regulatory approval process in ... |
August 26, 2013 — Boston Scientific Corp. has completed enrollment in the SuperNOVA trial – a global, single arm ...
August 19, 2013 — Cook Medical is again shipping its Zilver PTX drug-eluting peripheral stent to medical centers in the ...
August 16, 2013 — Patient enrollment has been initiated in a post-market registry for the Combo Dual Therapy Stent to ...
August 5, 2013 — AstraZeneca announced results from a new sub-analysis of the PLATO study that evaluated the incidence ...
August 1, 2013 — InspireMD Inc. said the first patient has been enrolled in the Master II IDE clinical trial to evaluate ...
July 18, 2013 — Abbott announced that it has entered into an agreement to purchase IDEV Technologies, a privately held ...


 September 25, 2013
September 25, 2013










