June 30, 2015 - The American College of Cardiology (ACC), Heart Rhythm Society (HRS) and Society for Cardiovascular ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.

June 24, 2015 - Scott & White Memorial – Temple for the first time implanted a new miniaturized, wireless monitoring ...
June 23, 2015 - Results from a clinical trial evaluating the Micra transcatheter pacing system (TPS), the world's ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

June 23, 2015 - Heart attack patients age 65 and older who have reduced heart function might still benefit from implante ...
June 22, 2015 - The current monitoring of patients with cardiac implantable electronic devices (CIEDs) may be ...
June 11, 2015 - C. Dorn Smith, M.D., vascular surgeon in Kingstree, South Carolina, was successful in using a new device ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
June 2, 2015 — The Valley Hospital has been selected as the United States Coordinating Center for the international ...
May 28, 2015 — CVRx Inc. announced that positive results from the 'Barostim Therapy for Heart Failure' randomized ...
There were several technology takeaway messages for the future of electrophysiology (EP) at the Heart Rhythm Society ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
May 21, 2015 — The Phase C results of the ProMRI Clinical Study were presented at the late-breaking clinical trial ...
May 21, 2015 — Important new data presented during the Heart Rhythm Society's (HRS) 36th annual Scientific Session ...
May 21, 2015 — Biotronik announced that since the launch of the ProMRI Eluna pacemaker system in late March 2015, 60 ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 21, 2015 — The Heart Rhythm Society (HRS) has released a first-of-its-kind expert consensus statement on three ...
May 21, 2015 — Janssen Pharmaceuticals Inc. and its development partner Bayer HealthCare announced results from the ...
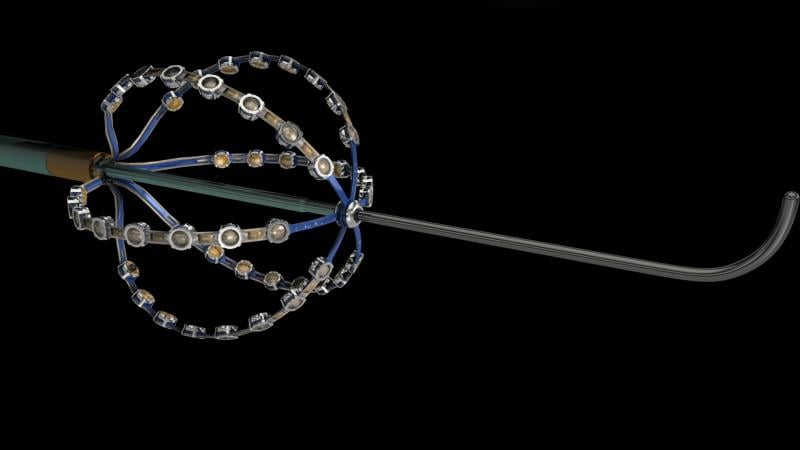
May 21, 2015 – A preliminary study of a new electrophysiology (EP) dipole density mapping system using a specialized ...

 June 30, 2015
June 30, 2015