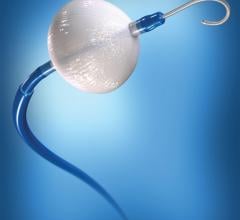
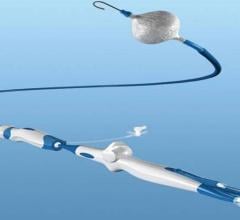
August 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and commercial availability of ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.

There is growing concern among patients and regulators that medical devices, especially implantable electrophysiology ...
August 7, 2017 — CardioFocus Inc. announced that its HeartLight Endoscopic Ablation System is being featured in three ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

The current generation of hemodynamic monitoring systems can help catheterization labs electronically document the ...
Aug. 2, 2017 — A new health economic analysis from the landmark FIRE and ICE Trial was published this week in the ...
Aug. 1, 2017 — The Japanese Ministry of Health, Labour and Welfare has granted Japanese market clearance for the ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

Aug. 1, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology ...
July 27, 2017 — There is good news when it comes to the heart’s sinoatrial node (SAN), the body’s natural pacemaker ...
July 24, 2017 — Medtronic plc recently announced first enrollments in the STOP AF First clinical trial. The trial will ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
July 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and availability of the Intica DX ...
July 14, 2017 — A review appearing in the July 18 issue of the Journal of the American College of Cardiology (JACC) disc ...
July 13, 2017 — Cardiologs Technologies SAS announced that it has received U.S. Food and Drug Administration (FDA) ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
July 13, 2017 — Experts in heart failure management gathered in June to discuss varying scientific evidence in their ...
July 12, 2017 — Cardiac Insight Inc., a U.S. developer of wearable medical devices and diagnostic software, announced ...
July 11, 2017 — iRhythm Technologies Inc. announced a collaboration with the Stanford Machine Learning Group that has ...


 August 21, 2017
August 21, 2017