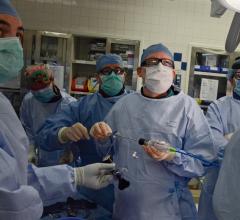
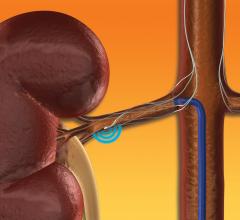
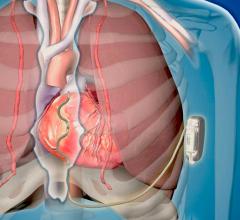
June 4, 2019 — Orchestra BioMed Inc. announced the presentation of two-year clinical data from the European Moderato I ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
Physicians use many strategies to better interface with patients and their families to try and explain in non-physician ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

This is a brief overview of updates on implantable cardioverter defibrillators (ICD), including new technology ...
May 23, 2019 – Investigators unveiled late-breaking clinical data from a first-of-its-kind physician sponsored clinical ...
May 17, 2019 — Digital healthcare company Murj announced the availability of the Murj Analytics software-as-a-service ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 17, 2019 — At the 40th annual Heart Rhythm Scientific Sessions, May 8-11 in San Francisco, Acutus Medical announced ...
May 16, 2019 — Preventice Solutions presented clinical data validating its BodyGuardian Remote Monitoring System with ...
May 16, 2019 — Johnson & Johnson Medical Devices Companies announced the launch of Biosense Webster Inc.’s Cartonet to ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
The Heart Rhythm Society (HRS) 2019 Annual Scientific Sessions represent an important annual event for electrophysi ...
May 16, 2019 — Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc.’s QDot Micro catheter ...
May 16, 2019 — CardioFocus Inc. announced the presentation of results from its pivotal confirmatory study evaluating the ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 16, 2019 — BioCardia Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Avance steerable ...

May 15, 2019 — The Heart Rhythm Society (HRS) had 21 late-breaking study presentations at the 2019 Heart Rhythm ...

May 15, 2019 — A new study shows electromechanical wave imaging is capable of localizing arrhythmias including atrial ...

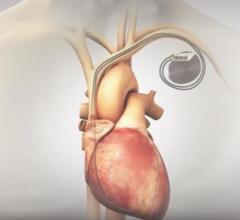
 June 04, 2019
June 04, 2019