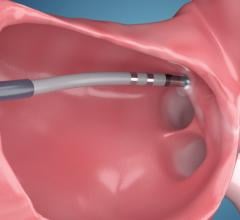
March 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
February 23, 2021 — Remote cardiac monitoring vendor RhythMedix released its next-generation RhythmStar wearable device ...

February 2, 2020 — The U.S. Food and Drug Administration (FDA) has identified a recent recall of the Boston Scientific ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
February 2, 2021 – Optimize EP, a digital health company focused on transforming cardiac care by improving the current ...

February 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
January 29, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Medtronic's DiamondTemp Ablation (DTA) system ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
In our most challenging and distressing days, it is nice to think about a post-COVID-19 world.
But while this ...

January 19, 2021 – With the postponement of non-essential elective surgeries and medical procedures in 2020 to conserve ...
January 8, 2020 — A new study published this week in HeartRhythm, the journal of the Heart Rhythm Society (HRS), found ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...

Here are the top 25 best performing articles on the Diagnostic and Interventional Cardiology (DAIC) website from 2020 ...
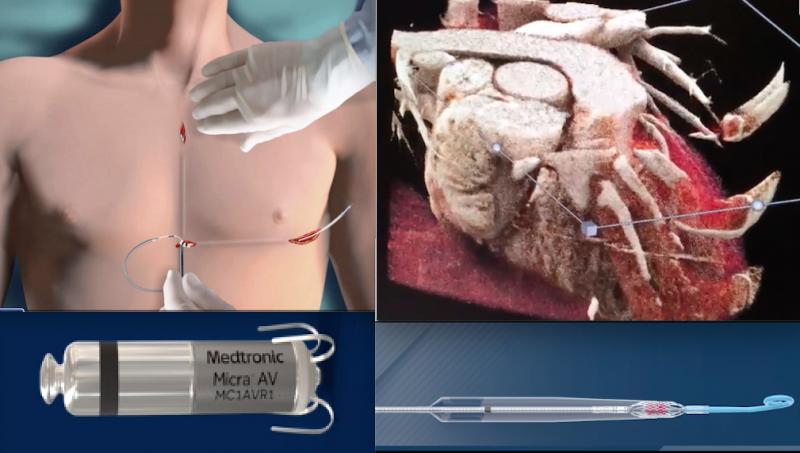
December 23, 2020 — Demonstrations of new technologies and explanations of how they work are among the most popular ...
December 23, 2020 — The American College of Cardiology (ACC) and the American Heart Association (AHA) has made two ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
December 22, 2020 — The U.S. Food and Drug Administration (FDA) cleared Biotronik's Vital Data Sensor, which identifies ...
Robert Kowal, M.D., chief medical officer of the Medtronic cardiac rhythm and heart failure division, said there has ...

COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...

 March 01, 2021
March 01, 2021