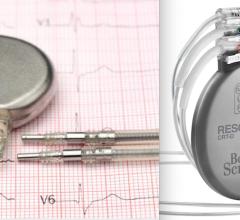
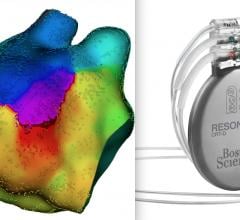
October 6, 2021 — Boston Scientific Corp. announced it entered into a definitive agreement to acquire Baylis Medical ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
October 6, 2021 – Biosense Webster announced the first cases using a radiofrequency balloon ablation catheter were ...
October 1, 2021 — The European Society of Cardiology (ESC) guidelines on cardiac pacing and cardiac resynchronization ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
Khaldoun Tarakji, M.D., MPH, associate section head, cardiac electrophysiology, Heart and Vascular Institute at ...
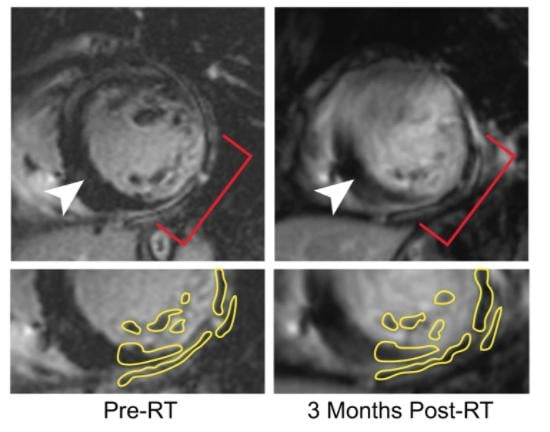
September 28, 2021 — New research from Washington University School of Medicine in St. Louis suggests that radiation ...
September 28, 2021 — Image-guided fibrosis ablation in addition to pulmonary vein isolation (PVI) does not improve ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
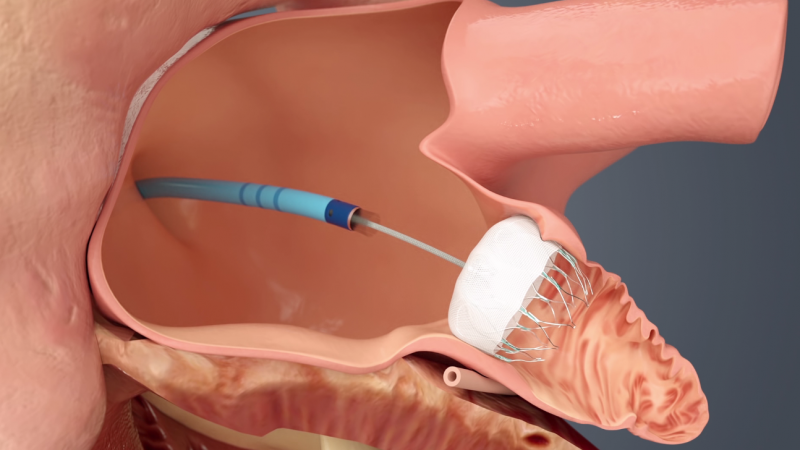
September 27, 2021 — Compared with men undergoing left atrial appendage occlusion (LAAO), women have a significantly ...

In the electrophysiology (EP) lab, hundreds of thousands of used devices are sent to reprocessors every year to get ...
September 13, 2021 — Ablation plus cardiac resynchronization therapy (CRT) is superior to pharmacological rate control ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
September 8, 2021 — Continuous heart rhythm monitoring – with anticoagulation if atrial fibrillation is detected – does ...
September 7, 2021 — Remote monitoring of implantable cardiac monitors (ICMs) is highly effective for early detection of ...
September 1, 2021 — Stereotaxis and Shanghai Microport EP Medtech Co., Ltd. announced a broad collaboration to advance ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
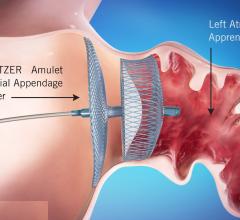
August 31, 2021 — Late-breaking data from a head-to-head clinical trial of the Amulet Left Atrial Appendage (LAA) ...
August 16, 2021 — The U.S. Food and Drug Administration (FDA) approved Abbott's Amplatzer Amulet Left Atrial Appendage ...
August 9, 2021 - In association with Heart Rhythm 2021, Biotronik today announced that the latest implantable cardiac ...


 October 06, 2021
October 06, 2021