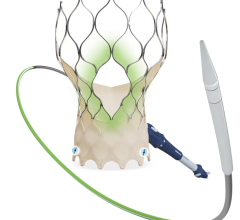
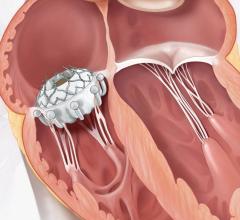
Illustration of the Watchman transcatheter left atrial appendage (LAA) occluder being deployed into the LAA.
September 27, 2021 — Compared with men undergoing left atrial appendage occlusion (LAAO), women have a significantly higher risk of in-hospital adverse events, according to a recently published review of more than 49,000 patients in the National Cardiovascular Data Registry LAAO Registry.[1] This data prompted the U.S. Food and Drug Administration (FDA) today to issue a safety letter to providers outlining the potential safety issues in women.
The FDA letter sent Sept. 27, informs healthcare providers of recent information about the potential for differences in procedural outcomes between women and men undergoing implant of a left atrial appendage occlusion device. The agency said it is evaluating a study published in August in the Journal of the American Medical Association (JAMA) Cardiology.[1] This analysis of real-world data in the National Cardiovascular Data Registry LAAO Registry from 49,357 patients undergoing LAAO procedures with the Watchman Left Atrial Appendage Closure device from 2016 to 2019 indicates that procedural outcomes, such as major adverse events and lengthened hospital stay, may be more common in women compared to men.
The causes for these differences are yet to be determined, the FDA said. The agency said it will work with the manufacturers of approved LAAO devices to evaluate information from several sources, including premarket studies, mandated post-market studies, and real-world data to provide additional information on this issue.
Despite the study raising a red flag, the FDA said it believes the benefits continue to outweigh the risks for approved LAAO devices when used in accordance with their instructions for use.
FDA Recommendations for Healthcare Providers on LAA Occlusion:
The FDA recommends that healthcare providers:
• Continue monitoring patients who have been treated with LAAO devices per the current standard of care.
• Discuss the risks and benefits of all available options for stroke prevention in patients with atrial fibrillation as part of shared clinical decision-making.
• Report any adverse events or suspected adverse events experienced with the use of LAAO devices. Voluntary reports can be submitted through MedWatch, the FDA Safety Information and Adverse Event Reporting program. Device manufacturers and user facilities must comply with the applicable Medical Device Reporting (MDR) regulations. Healthcare personnel employed by facilities that are subject to FDA's user facility reporting requirements should follow the reporting procedures established by their facilities. Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices.
FDA Will Be Watching Data on Two Transcatheter LAA Occluders
LAAO devices are implanted in the heart and are intended to reduce the risk of thromboembolism from the left atrial appendage (LAA) in patients with non-valvular atrial fibrillation. The device mechanically occludes the LAA to prevent LAA thrombus from entering the systemic circulation. The manufacturers of the currently approved and marketed LAAO devices in the U.S. are Abbott Amplatzer Amulet device and Boston Scientific Watchman and Watchman FLX devices.
Study Showing Safety Concern in LAAO Registry Data
The recent article by Darden et al. presented an analysis of 49,357 patients from the LAAO registry (41.3% women and 58.7% men) treated with the Watchman device between 2016 to 2019. The authors found a statistically significant higher rate of adverse procedural events in women compared to men including any adverse events (6.3% vs. 3.9%, p<0.001), any major adverse events (4.1% vs 2%, p<0.001), and hospital stay longer than one day (16% vs 11.6%, p<0.001).
Among specific procedural adverse events, the rates of pericardial effusion requiring percutaneous drainage was 1.2% vs. 0.5% and major bleeding was 1.7% vs. 0.8% in women vs. men, respectively. Procedure-associated death was 0.3% in women and 0.1% in men.
The FDA said it recognizes the limitations of these data, including that the study was not randomized, only included one LAAO device (the first-generation Watchman device), and did not include longer-term outcomes beyond in-hospital events. However, the analysis provides results from a large registry of patients treated with LAAO implants in the U.S.
Registries are real-world databases that can be used to track how patients respond to particular medical devices and are one source the FDA uses to monitor post-market device safety and effectiveness. Because the National Cardiovascular Data Registry LAAO Registry captures a large number of patients undergoing LAAO procedures, the number of patients included in this analysis was much larger than the number of patients that were included in the premarket studies of the Watchman device that supported device approval, and the premarket studies for the other approved LAAO devices.
FDA Working With LAA Occluder Vendors to Gather More Safety Data
The FDA said it is working with the manufacturers to evaluate the potential issue of sex differences in procedural outcomes with LAAO devices, including a review of available premarket and post-approval study data, and other available real-world post-market data sets. In addition, the FDA will work with the device manufacturers, investigators, and the LAAO Registry to try to identify the causes of procedural outcome differences between women and men.
The FDA said it will communicate with the public if new or additional information becomes available.
Related LAA Occluder Content:
FDA Clears Abbott Amplatzer Amulet LAA Occluder to Reduce Stroke in People With Atrial Fibrillation
VIDEO: Overview of LAA Occlusion Using the Watchman FLX — Interview with Devi G. Nair, M.D.
NCDR LAAO Registry Shows Left Atrial Appendage Occlusion Associated With Low Rate of Stroke
Occluding the Left Atrial Appendage (LAA)
VIDEO: Comparison Between Watchman vs. Amulet LAA Occluders — Interview with Ashish Pershad, M.D.
First-Of-Its-Kind, No-Implant LAA Occluder Noted for Innovation at 2019 ICI Meeting
VIDEO: Overview of Left Atrial Appendage (LAA) Closure Technology and New Innovations — Interview with Horst Sievert, M.D.
Amplatzer Amulet LAA Occluder Will be Compared to Anticoagulants in CATALYST Trial
VIDEO: Gender Differences in Diagnosing Heart Disease in Women — Interview with Doreen DeFaria Yeh, M.D.
Find more new on Left Atrial Appendage (LAA) Occluders
Reference:


 July 08, 2024
July 08, 2024