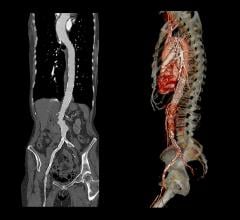
January 29, 2020 – The presence of a blood clot on the wall of the aorta in people with abdominal aortic aneurysms (AAA) ...
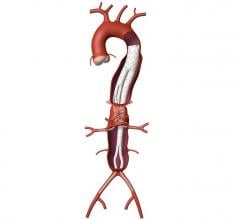
Endovascular Aortic Repair
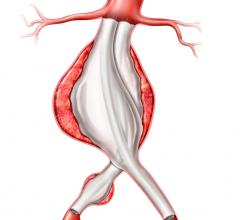
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
November 7, 2019 — The Endospan Ltd. Nexus aortic arch stent graft is a CE mark–approved, off-the-shelf system for ...
October 9, 2019 — Medtronic plc announced it has received Breakthrough Device designation from the U.S. Food and Drug ...
October 8, 2019 — PQ Bypass Inc. announced it has received full approval of its investigational device exemption (IDE) ...
July 10, 2019 — W. L. Gore & Associates Inc. (Gore) announced the first U.S. implant of the Gore Tag Conformable ...
May 15, 2019 — W. L. Gore & Associates Inc. (Gore) announced the U.S. Food and Drug Administration (FDA) has granted ...
April 15, 2019 — Biotronik began its U.S. commercial launch of the PK Papyrus covered coronary stent system for use in ...
Here is what I thought were the top five most important take-away presentations from the 2019 Society of Thoracic ...
February 14, 2019 — Abdominal aortic stent grafts will remain the largest segment in the global aortic stent graft ...
February 7, 2019 — Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for the Valiant ...
January 7, 2019 — Endologix Inc. announced that in order to ensure optimal outcomes for patients, unrestricted sales and ...
November 21, 2018 — New research in the December edition of the Journal of Vascular Surgery suggests significant ...
October 8, 2018 — Endologix Inc. received notice that the U.S. Food and Drug Administration (FDA) has classified a ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
August 23, 2018 — W. L. Gore & Associates Inc. (Gore) announced U.S. Food and Drug Administration (FDA) 510(k) clearance ...


 January 29, 2020
January 29, 2020