January 25, 2016 — The suspension of the medical device excise tax will have positive consequences for the U.S. medical ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
This case study is from the Cardiac Imaging Department, Hospital Clinic, Barcelona, Spain. A woman in her early 60s came ...
GE Healthcare is taking the next leap in image quality performance, quantification and workflow with the introduction of ...
January 20, 2016 — On the 100th anniversary of the Endurance expedition to Antarctica led by Sir Ernest Shackleton ...
January 20, 2016 — Patients enrolled in high-deductible health insurance plans have lower rates of use and lower costs ...
At the beginning of each year, I always try to determine what the next big cardiovascular technology advances to watch ...
January 18, 2016 — Medtronic plc announced that the IN.PACT Admiral drug eluting balloon (DEB) has received CE ...
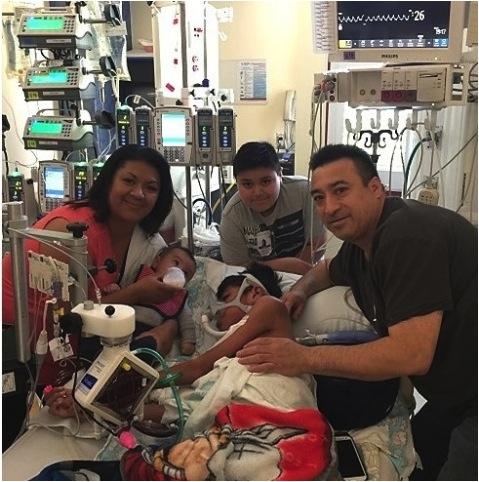
January 15, 2016 — 2016 is starting off a whole lot better than last year for 14-year-old Oswaldo Jimenez of Salem, Ore ...
January 15, 2016 — Teleflex Inc. has acquired privately held Nostix LLC, developer of innovative tip confirmation ...
January 15, 2016 — Patients between the ages of 40 and 70 who undergo aortic valve replacement (AVR) may fare better ...
January 15, 2016 — CardioKinetix Inc. announced that it has enrolled more than half of the subjects in its pivotal ...
January 15, 2016 — The Centers for Medicare and Medicaid Services (CMS) announced Monday that it will be ending the ...

January 15, 2016 — The U.S. Food and Drug Administration (FDA) has given its approval for an expanded indication study ...
January 14, 2016 — Cornell biomedical engineers have discovered natural triggers that could reduce the chance of life ...
January 14, 2016 — Penumbra Inc. announced the U.S. launch of its new POD Packing Coil, designed as a complementary ...

 January 25, 2016
January 25, 2016