Contrast Media
Contrast media, also called contrast agents, are used to enhance the blood and perfusion in tissues.This includes iodine based contrast for on computed tomography (CT), gadolinium based agents for MRI and lipid bubble contrast agents used in ultrasound.

January 27, 2015 — Ortho-Clinical Diagnostics Inc. announced the nationwide availability to hospitals of the Nephrocheck ...
January 6, 2015 — Bayer HealthCare announced that the U.S. Food and Drug Administration (FDA) has approved Gadavist ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
December 23, 2014 — Bayer unveiled version 2.5 of its Radimetrics Enterprise Platform at the 2014 Radiological Society ...
December 16, 2014 — Data from a Bayer study in children less than 2 years of age (infants) was presented at the 2014 ...
November 24, 2014 — Bracco Diagnostics Inc. announced the availability of its Isovue (iopamidol injection) Imaging Bulk ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 14, 2014 — The U.S. Food and Drug Administration (FDA) approved Lumason (sulfur hexafluoride lipid microsphere) ...
September 25, 2014 — Mallinckrodt announced the U.S. launch of the OptiSync data management system as a new technology ...
As part of the Consolidated Appropriations Act of 2018, pass-through payment status for LUMASON® (sulfur hexafluoride ...

September 10, 2014 — According to new research performed at the Mayo Clinic, iodine-based contrast material injected ...
August 11, 2014 — Imaging experts from the American Society of Echocardiography (ASE) released a paper, "Guidelines for ...
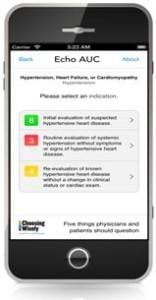
July 3, 2014 — The American Society of Echocardiography Foundation (ASEF) launched the second version of its free mobile ...

April 4, 2014 — GE Healthcare announced results from a new clinical study, which showed that patients receiving ...

March 11, 2014 — Guerbet announced a worldwide launch of FlowSens, a contrast agent injection solution used in X-ray med ...

 February 10, 2015
February 10, 2015