February 15, 2017 — Deep learning, also known as artificial intelligence, will increasingly be used in the ...
February 15, 2017 — Better hospital supply chain management leads to better quality of care and supports patient safety ...
In the current era of healthcare reform and the push toward evidence-based medicine to both lower costs and improve ...
Stents were the key focus of interventional cardiology for more than 20 years, but the focus has changed in recent years ...
February 13, 2017 — Vital Images Inc. announced that it will be demonstrating new image sharing capabilities for current ...
February 10, 2017 — With the Healthcare Information and Management Systems Society’s annual meeting (HIMSS17) scheduled ...

In the past few years there have been a number of device therapies developed to treat heart failure (HF). This is partly ...
February 9, 2017 — Vital Images Inc. will feature its Vitrea Modular Enterprise Imaging interoperability solutions at ...
February 9, 2017 — eHealth Technologies and HealtheConnections, a Syracuse-based regional health information ...
February 9, 2017 — Charleston Area Medical Center (CAMC) has documented reduced readmissions for congestive heart ...
February 7, 2017 — Healthcare analytics company Innovaccer Inc. announced the launch of its holistic MIPS Platform ...
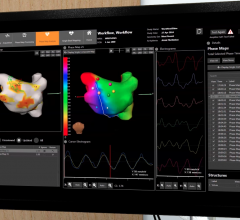
This video, provided by Medtronic, demonstrates the CardioInsight electro-anatomical mapping system. It was cleared by ...
February 1, 2017 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the CardioInsight ...
January 31, 2017 — The American College of Cardiology (ACC) issued a statement against President Donald Trump’s recent ...

The movement from paper electrocardiogram (ECG) review to electronic ECG management systems in the past decade has ...


 February 15, 2017
February 15, 2017