I attended the American Society of Echocardiography (ASE) 2018 meeting in June and have the following takeaways on ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
DAIC Editor Dave Fornell highlights some of the most innovative new technology on the show floor of the American Society ...

Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Rebecca Hahn, M.D., professor of medicine and director of interventional echocardiography, Columbia University Medical ...
July 3, 2018 — HeartFlow Inc. announced that UnitedHealthcare now covers the HeartFlow FFRct Analysis, extending access ...
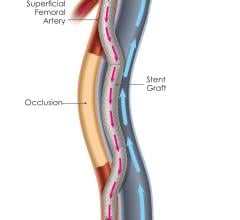
July 2, 2018 — The PQ Bypass Detour System showed promising 12-month durability for patients with extremely long ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
This is a demonstration of the the Philips TrueVue photo-realistic rendering and lighting source technology. This ...
June 25, 2018 — Computed tomography (CT) angiography is a useful adjunct to autopsy that is likely to increase the ...

About 50 percent of patients in cardiogenic shock do not survive, and account for about 90,000 heart attack patients a ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
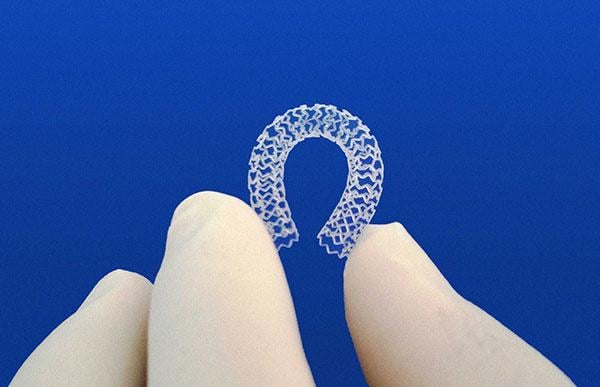
June 19, 2018 – Amaranth Medical offered new details about its 85-micron Defiance bioresorbable scaffold (BRS) recently ...
June 15, 2018 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for 200mm and 250mm lengths ...
June 14, 2018 — The U.S. Food and Drug Administration (FDA) released the following two draft guidance documents:
- Coro ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 13, 2018 — Henry Ford Hospital is one of 17 U.S. trial sites using a catheter-based procedure approved in Europe to ...
June 8, 2018 — Whole-body magnetic resonance angiography (MRA) found a surprisingly high prevalence of atherosclerosis ...
June 7, 2018 — The first clinical cases have been completed where the Emboliner Embolic Protection Catheter was used ...


 July 10, 2018
July 10, 2018