April 15, 2019 — For the first time in the United States, doctors with the American Heart Association (AHA) have ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

As we strive to process today’s successive news cycles involving negative reports about immigration, it is easy for many ...
April 15, 2019 – Intact Vascular Inc. received U.S. Food and Drug Administration (FDA) market clearance for the Tack ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
April 15, 2019 — Biotronik began its U.S. commercial launch of the PK Papyrus covered coronary stent system for use in ...
April 10, 2019 — According to a new research study, straight tip guidewires, with a current share of more than a third ...
April 9, 2019 — A program designed to help heart attack patients with the transition from hospital to outpatient care ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
April 8, 2019 — Rolling Stones frontman Mick Jagger is reportedly recovering after undergoing a transcatheter aortic ...
April 8, 2019 — TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) granted premarket approval for ...
April 5, 2019 — Three years ago this week, Abiomed's Impella heart pump received U.S. Food and Drug Administration (FDA) ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
April 4, 2019 — The U.S. Food and Drug Administration (FDA) granted Omega Medical Imaging 510(k) clearance to offer ...
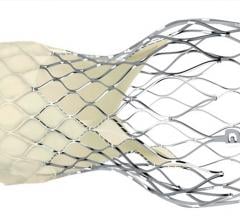
April 3, 2019 – The ACC.19 late-breaking landmark Evolut Low Risk Trial compared the minimally invasive Evolut ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently granted an additional indication to Bard Peripheral ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently cleared Bard Peripheral Vascular's Venovo Venous ...
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...
April 3, 2019 — Medtronic's Resolute Integrity Zotarolimus-eluting Coronary Stent System received an additional U.S ...


 April 15, 2019
April 15, 2019