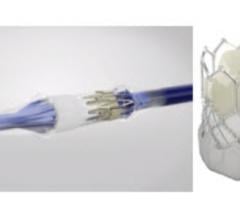
August 22, 2019 — Edwards Lifesciences is recalling the Sapien 3 Ultra delivery system after receiving reports of burst ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
August 21, 2019 — Increasing incidence of cardiovascular diseases worldwide is creating growth opportunities for the ...
August 21, 2019 — Jackson Memorial Hospital in Miami celebrated the opening of two newly renovated cardiac ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
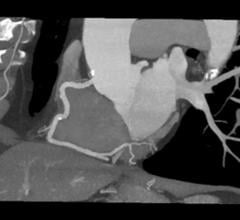
August 16, 2019 — In one coordinated move, the U.S. Food and Drug Administration (FDA) opened use of transcatheter ...
August 14, 2019 — Ancora Heart Inc. announced the first patient was enrolled in the CorCinch EU study, a European multi ...
August 14, 2019 — Concept Medical Inc. (CMI) has been granted "Breakthrough Device Designation" from the U.S. Food and ...
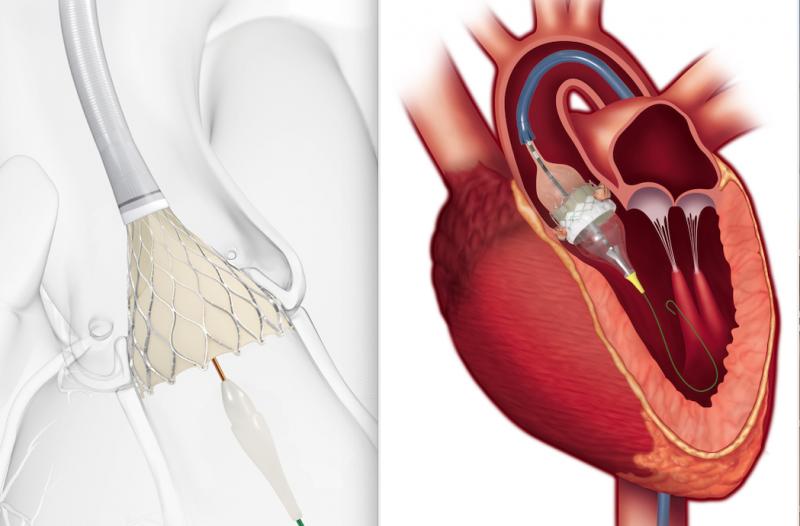
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
August 13, 2019 — A simple change in the way health professionals track their patients’ progress has brought improved ...
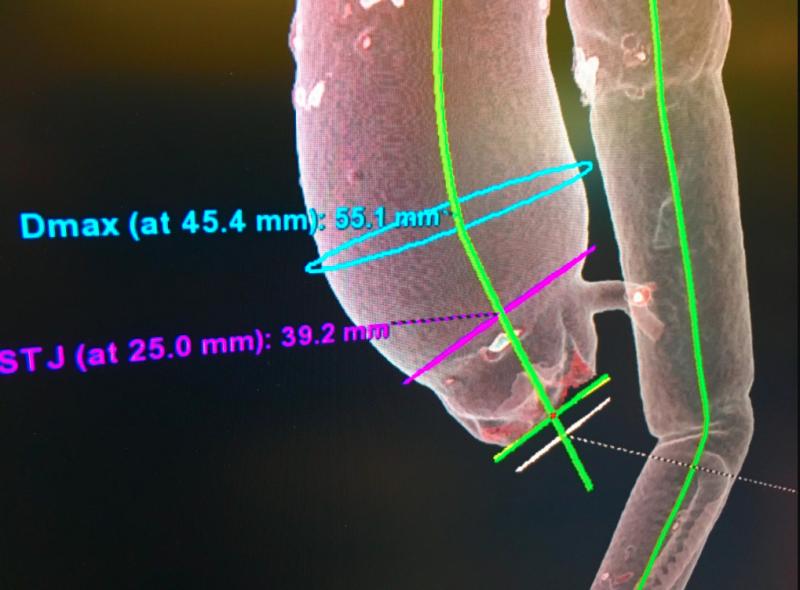
As we usher in a new era for treating patients with aortic stenosis, we have been reflecting on the evolution of the ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 8, 2019 — Corindus Vascular Robotics announced it has entered into a definitive merger agreement to be acquired ...
August 6, 2019 — Cardiovascular Systems Inc. (CSI) has acquired the Wirion Embolic Protection System and related assets ...
August 6, 2019 — When used with a common heart scan, machine learning, a type of artificial intelligence (AI), does ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

New technologies have been developed that may replace the traditional pressure wires and adenosine to assess the fractio ...
August 2, 2019 — B. Braun Interventional Systems Inc. (BIS) announced the U.S. Food and Drug Administration (FDA) has ...

Guidewire engineering has become more advanced over the past decade as interventional cardiologists have advanced their ...

 August 22, 2019
August 22, 2019