October 29, 2019 — Siemens Healthineers completed the acquisition of 100 percent of Corindus Vascular Robotics Inc ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Office based labs (OBLs) and ambulatory surgery centers (ASCs) require a fresh perspective from imaging vendors. The Alp ...
October 28, 2019 — The Gore Cardioform ASD Occluder received European CE mark clearance in early October. The device ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
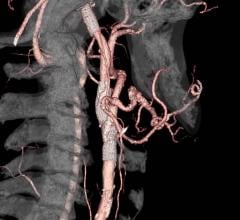
October 28, 2019 — Leaders within University Hospitals and the Harrington Heart and Vascular Institute had a vision to ...
October 28, 2019 — Nearly two years of real-world outcomes data on Abiomed’s Impella RP heart pump shows that when ...
October 23, 2019 — BioCardia announced the U.S. commercial availability of its Avance Bi-Directional Steerable ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 22, 2019 — OmniVision Technologies Inc. announced that its OV6948 camera module is the winner of the Guinness ...
Sunil Rao, M.D., chief of cardiology, Durham VA Health System and a professor at Duke University, and Prashant Kaul, M.D ...
October 21, 2019 — Cardiovascular Systems Inc. recently announced U.S. Food and Drug Administration (FDA) premarket ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 18, 2019 — An algorithm developed by faculty at The University of Texas Health Science Center at Houston ...
October 18, 2019 — Robocath recently announced the success of its first two coronary angioplasties performed with ...
October 18, 2019 — A new study published in Clinical Cardiology[1] introduces the North Indian (NORIN) ST‐Segment ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Ashish Pershad, M.D., chief of interventional cardiology, Banner University Medical Center, Phoenix, explains the trend ...
Roxana Mehran, M.D., FACC, FACP, FCCP, FESC, FAHA, FSCAI, professor of medicine and director of interventional ...
Jeffrey J. Popma, M.D., director of interventional cardiology clinical services at Beth Israel Deaconess Medical Center ...


 October 29, 2019
October 29, 2019