Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
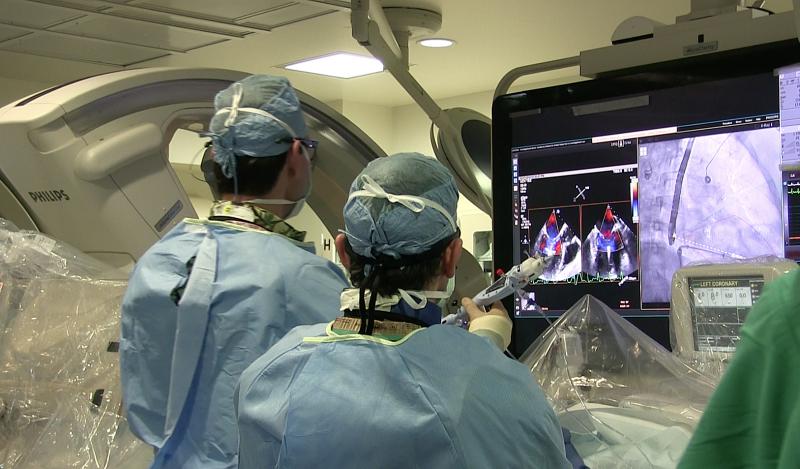
December 30, 2019 — Merit Medical Systems Inc., a leading manufacturer of proprietary devices used primarily in ...
December 30, 2019 — Most patients do not understand or recall information given to them before heart procedures. For ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

December 27, 2019 — The U.S. Food and Drug Administration (FDA) has approved two applications for the first generics of ...
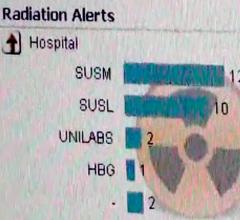
Mahadevappa Mahesh, Ph.D., chief of medical physicist and professor of radiology and medical physics, Johns Hopkins ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
DAIC Editor Dave Fornell and Imaging Technology News (ITN) Consulting Editor Greg Freiherr offer a post-game report on ...
December 18, 2019 — Cook Medical initiated a recall of its CrossCath Support Catheters in November, which the U.S. Food ...

December 16, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

December 16, 2019 — The U.S. Food and Drug Administration (FDA), on Dec. 13, approved the use of Vascepa (icosapent ...
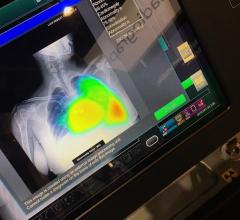
November 27, 2019 — CAE Healthcare will showcase its mixed reality training solutions for practicing physicians and ...
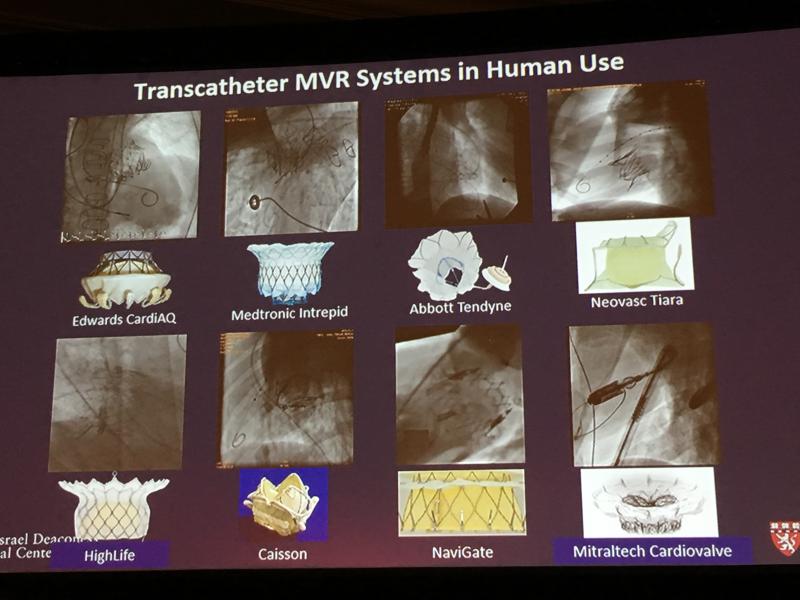
The overwhelming success story for transcatheter aortic valve replacement (TAVR) moving from a science project to ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
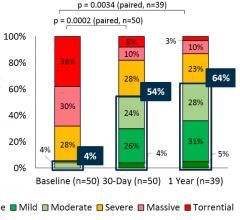
November 26, 2019 — The preliminary one-year results of the TRILUMINATE Pivotal Study for Abbott's TriClip device, a ...
November 21, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic In.Pact AV drug-coated balloon ...
Now, more than ever, the field of cardiology needs women.
But as the national need for more cardiologists overall ...

 December 30, 2019
December 30, 2019