Mahadevappa Mahesh, Ph.D., chief of medical physicist and professor of radiology and medical physics, Johns Hopkins ...
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Mahadevappa Mahesh, Ph.D., chief of medical physicist and professor of radiology and medical physics, Johns Hopkins ...
DAIC Editor Dave Fornell and Imaging Technology News (ITN) Consulting Editor Greg Freiherr offer a post-game report on ...
December 18, 2019 — Cook Medical initiated a recall of its CrossCath Support Catheters in November, which the U.S. Food ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

December 16, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...

December 16, 2019 — The U.S. Food and Drug Administration (FDA), on Dec. 13, approved the use of Vascepa (icosapent ...
November 27, 2019 — CAE Healthcare will showcase its mixed reality training solutions for practicing physicians and ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
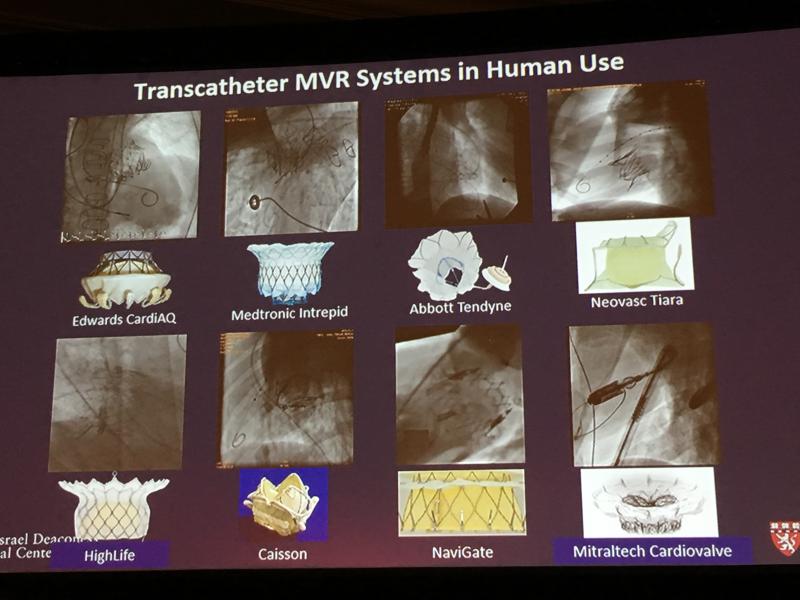
The overwhelming success story for transcatheter aortic valve replacement (TAVR) moving from a science project to ...
November 26, 2019 — The preliminary one-year results of the TRILUMINATE Pivotal Study for Abbott's TriClip device, a ...
November 21, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic In.Pact AV drug-coated balloon ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Now, more than ever, the field of cardiology needs women.
But as the national need for more cardiologists overall ...


November 19, 2019 — After 12 years of collecting data, the results of the landmark ISCHEMIA (International Study of ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
November 19, 2019 — Since Maquet/Datascope first recalled all of its intra-aortic balloon pumps (IABP) due to reports ...

November 19, 2019 — Here is a list of the key late-breaking clinical study presentations and links to the results at the ...
November 7, 2019 — The ClotTriever Outcomes (CLOUT) Registry is evaluating real-world patient outcomes following ...