February 13, 2020 — The U.S. Food and Drug Administration (FDA) has issued its final guidance on "Peripheral Vascular ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 12. 2020 — Philips announced a new randomized controlled trial to assess patient outcomes after receiving a ...
February 12, 2020 — The University of Wisconsin (UW) Health’s University Hospital in Madison, Wis., recently became the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

Cardiovascular diseases (CVDs) are among the leading causes of death across the globe. For patients suffering from high ...
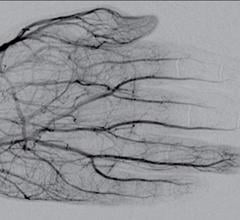
February 11, 2020 — An ahead-of-print article in the April issue of the American Journal of Roentgenology (AJR) ...

DAIC Editor Dave Fornell recently took a tour of the Abiomed Impella production line at its headquarters in Danvers ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Interview with James Udelson, M.D., chief of the division of cardiology at Tufts Medical Center, Boston. The hospital ...
February 7, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the Aria ...
Interview with Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 4, 2020 — For some patients, kidney cancer can be effectively treated without surgery, according to the Society ...
February 3, 2020 — The U.S. Food and Drug Administration (FDA) has approved a the CATALYST trial designed to assess ...
February 3, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) m ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 31, 2020 — A new study from the Smidt Heart Institute at Cedars-Sinai and other centers nationwide shows that ...
January 29, 2020 – Nuance Communications Inc. introduced Nuance Cardiovascular CAPD, a new computer-assisted physician ...
January 31, 2020 – The Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) released new reporting ...


 February 13, 2020
February 13, 2020