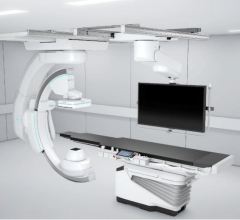
The Boston Scientific Eluvia self-expanding, drug-eluting, peripheral stent.
Cardiovascular diseases (CVDs) are among the leading causes of death across the globe. For patients suffering from high-risk cardiovascular disease, implementation of stents has become a key device in the treatment of obstructive coronary disease.
Stents are used most often to alleviate symptoms in select patients suffering from obstructive coronary artery diseases. Studies suggest that more than 2 million people across the globe get stents implanted each year. The stent industry players across the globe are making persistent efforts to produce innovative and groundbreaking stent technologies that will help them secure a leading position in the domain.
Strategic Positioning by Stent Manufacturers
The top companies operating in stents market are Biosensors, Stryker, Abbott, Boston Scientific, B. Braun, Medtronic, Terumo and Biotronik. Increasing number of cardiovascular surgeries performed in tandem with minimally invasive techniques are stimulating stent industry growth.
Key stent makers are implementing strategies to gain a stronger foothold in the industry. An example is Boston Scientific, which launched its self-expanding Eluvia drug-eluting stent (DES) system for peripheral artery disease (PAD) in 2018. This launch served a dual purpose, it helped the company expand its PAD portfolio, as well as using a new drug-polymer combination to offer sustained release of the drug paclitaxel for a one-year timeframe, designed to prevent tissue regrowth that might otherwise block the stented artery. This was a new advance in the treatment of peripheral artery disease.
In the Asia-Pacific (APAC) region, Indian stent manufacturer SMT (Sahajanand Medical Technologies) announced its acquisition of majority stake in Brazilian sales and marketing firm Zarek Distribuidora De Produtos Hospitalares. Considering that Brazil is among the most sought-after markets for interventional cardiology across the globe, this endeavor is estimated to prove immensely beneficial for SMT in coming years. The alliance will allow SMT to establish a strong overseas presence not just in Brazil, but also other Latin American countries like Bolivia, Colombia, Venezuela and Argentina to name a few. This indicates a positive global growth trajectory for the local stent manufacturer.
Developments in APAC Stent Market
Globally, about 13 million stents were sold in 2018. The primary strategy adopted by local players in China and India is low cost of stents. For instance, in 2018, the Indian National Pharmaceutical Pricing Authority (NPPA) revised the price of drug eluting stents downwards by reducing the prices down by about $33.
Drug eluting stents are used in about 95 percent of all percutaneous coronary intervention (PCI) cases in India. The price capping reduced the cost associated with majority of stents. Stents that are made indigenously perform on par with other stents manufactured by foreign multi-national corporations.
SMT has been noted by some key leaders in interventional cardiology, including Martin Leon, M.D., for its development of devices that appear to be on par with those made by some of the key American and European vendors. This has been reinforced by clinical data. SMT's ultra-thin Supraflex DES maintained numerically lower outcomes at two years of follow-up in a randomized trial against Abbott’s Xience stent, according to results from the TALENT trial presented at Transcatheter Cardiovascular Therapeutics (TCT) 2019. Patients who got the Supraflex stent had 6.9 percent target lesion failure occur at 720 days, versus 7.9 percent percent of Xience recipients.
Additionally, Indian manufacturers offer comparable margins to hospitals, even with reduced prices of stents, thereby increasing the market share of local manufacturers significantly.
Keeping in line with the benchmark set by the market-leading Xience stent, industry players have rallied to produce innovative and efficient stents. Companies are engaging in various studies and clinical trials, and seeking market approval for products that could meet, or possibly even transcend the high standards set by the Xience stent.
For instance, Biotronik has recently revealed that its new Orsiro DES has received approval for sale by the National Medical Products Administration in China. This approval means that the company will be able to make the stent available to Chinese medical practitioners and patients in a matter of months.
Likewise, Japanese firm OrbusNeich Medical K.K has also announced that the Ministry of Health Labor and Welfare (MHLW) in Japan has approved the company’s Combo Plus Coronary Stent for the Shonin market. This stent is touted as the first DES to combine the exclusive endothelial progenitor cell or EPC capture technology with a biodegradable matrix polymer-delivered abluminal sirolimus drug elution, achieving complete dissolution within 90 days.
What is on the Horizon for the Stent Market?
The quest for achieving a strong foothold in the global stent market is encouraging stent makers and medical device manufacturers to invest in innovations and research and enhance their portfolios.
For instance, Meril Life Sciences Pvt. Ltd has created what it claims to be India’s first locally produced bioresorbable vascular scaffold (BVS). The MeRes100 stent is touted as one of the company’s thinnest stents. The advantage of a bioresorbable stent is that it does not leave behind a permanent metallic implant, eliminating the cause of inflammation in some patients that leads in-stent restenosis. It also reduces the need for long-term antiplatelet therapy and preserving future treatment options for bypass surgery or another stent.
The product has shown immense promise, so much so that the government is now considering lifting the price caps introduced two years prior and exempting new generation stents from the directive. A new committee has been assembled on the National List of Essential Medicines to examine this issue. Spearheaded by Indian Council of Medical Research Director-General Balram Bhargava, the committee will deliberate on whether all drug-eluting stents display the same characteristics or require a new category, according to industry experts.
ETH Zurich researchers have introduced a new method to create malleable microstructure production using indirect 4-D printing. This new technology may help create vascular stents nearly 40 times smaller than previous versions. The fourth dimension is achieved through the stent’s shape-memory characteristics that enable the device to remember and return to its original shape when warm. Although still far away from human studies, these stents hold great potential for future implementation in previously unchartered medical aspects, like widening life-threatening urinary tract constrictions in unborn fetuses.
Global Market Insights Inc. has a market report dedicated to global stent industry, available at: www.gminsights.com/industry-analysis/stent-market.
What Are Stents? – An Overview
A stent is a small wire mesh tube that is inserted into blocked passageways to keep them open and facilitate easy flow of fluids. Depending on its placement, a stent can clear blockages or strengthen weak and narrow arteries, support blood vessels in the brain or stabilize bile and urine ducts.
Stents are generally made from either plastic or metal. Stent grafts, which are used in the aorta to seal aortic aneurisms, use a self-expanding stent framework and are covered in a specialized material to seal off the aneurysm.
Drug-eluting stents (DES) are used to help reduce neointimal hyperplasia scar tissue formation that occurs in some patients, whose bodies react to the stent as a foreign body. DES have helped reduce restenosis rates.
About Author: Saloni Walimbe, Research Content Developer at Global Market Insights (GMI). Saloni is specializes in content creation by penning down insightful articles relating to global industry trends, business, trade and finance. With an MBA in marketing, she has spent two years as a content writer.
Related Stent Content:
New Directions and Trends in Coronary Metallic Stents
New Peripheral Stent Technology
Coronary Stent Market Characterized by New Drug-eluting Stents
The Next Step in Bioresorbable Stent Technologies


 May 01, 2025
May 01, 2025