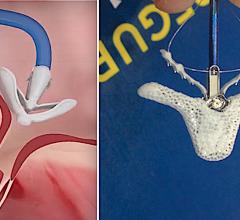
Devi G. Nair, M.D., FHRS, director of cardiac electrophysiology, St. Bernards Heart and Vascular Center, Jonesboro, Ark ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 24, 2020 — CeloNova BioSciences Inc. announced it successfully completed enrollment of the COBRA REDUCE randomized ...
July 22, 2020 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific's next generation Watchman ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
July 21, 2020 — Interventional cardiology is ranked as the occupation with the highest level of radiation exposure in ...
July 20, 2020 – BD (Becton, Dickinson and Company) recently completed the acquisition of Straub Medical AG, a privately ...
July 16, 2020 – The U.S. Food and Drug Administration (FDA) approved one-way digital data streaming during patient ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
Richard Botto, CVT, RCSA, chief cardiovascular technologist, division of cardiology, cardiac cath lab, offers an ...
July 13, 2020 — Edwards Lifesciences Corp. and Abbott Vascular announced the two companies have reached an agreement to ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
The Vieussens’ arterial ring (VAR) is a connection between the conus artery and the left anterior descending (LAD) ...

For a cardiovascular service line leader, addressing challenges in an evolving healthcare climate is a constant ...
July 9, 2020 — The Society for Vascular Surgery (SVS) has released a clinical competence statement on training and ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 9, 2020 – Acist Medical Systems Inc., a Bracco Group Company, announced the global launch of its Acist Navvus II ...
July 9, 2020 – The Minneapolis Heart Institute Foundation (MHIF) is conducting additional research on a novel hydrogel ...
July 8, 2020 – Philips Healthcare and Leeds Teaching Hospitals NHS Trust announced a seven-year managed service ...


 July 24, 2020
July 24, 2020