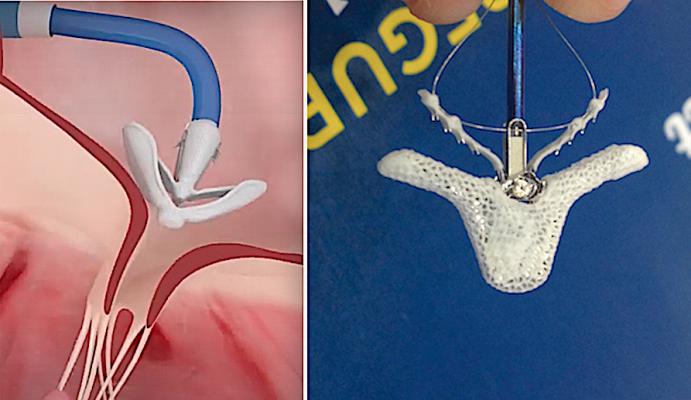
July 13, 2020 — Edwards Lifesciences Corp. and Abbott Vascular announced the two companies have reached an agreement to settle all outstanding patent disputes between the companies in cases related to transcatheter mitral and tricuspid repair products.
The agreement will result in the dismissal of all pending cases or appeals in courts and patent offices worldwide, and includes a provision that the parties will not litigate patent disputes with each other in the field of transcatheter mitral and tricuspid repair and replacement products for the 10-year duration of the agreement. The injunctions currently in place against the sale of Edwards' transcatheter mitral and tricuspid repair system will be lifted.
In connection with this agreement, Abbott will receive a one-time payment and ongoing payments based on the sales of the Edwards Pascal transcatheter valve repair system through 2025, as well as a potential sales milestone payment in 2026. The Pascal is very similar in design to the Abbott MitraClip device that is used for transcatheter mitral and tricuspid valve leaflet repairs.
Edwards said considers this agreement a positive development, as it allows the company to fully dedicate time and resources to helping patients.
Details of the settlement are confidential.
Related Transcatheter Mitral Valve Device Content:
Developments in Transcatheter Mitral Valve Replacement
Transcatheter Tricuspid Valve Repair Clip Device Cleared in Europe
New Study Looks at MitraClip use in Moderate Surgical Risk Patients
Edwards Pascal Tricuspid Valve Transcatheter Repair System Approved in Europe
Edwards Announces Research Milestones for Pascal Transcatheter Mitral Valve Program


 November 14, 2025
November 14, 2025 









