Juan F. Granada, M.D., CEO of the Cardiovascular Research Foundation (CRF) explains structural heart innovations and new ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
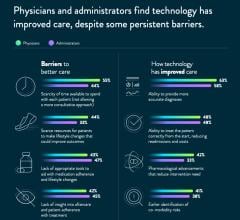
September 14, 2020 — New global research released by Abbott takes a deep dive into the barriers of cardiovascular ...
September 14, 2020 — Avinger received U.S. Food and Drug Administration (FDA) 510(k) clearance from the FDA for its ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Nick West, M.D., chief medical officer for Abbott, explains the details from a survey of 1,400 patients, physicians and ...
September 11, 2020 — The National Institutes of Health (NIH) has launched two of three adaptive clinical trials ...
September 11, 2020 – Medtronic announced U.S. Food and Drug Administration (FDA) approval of an early feasibility study ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Atlantic Health System’s Morristown Medical Center has opened one of the region’s first radial lounges in its Gagnon ...
September 5, 2020 – The TIDES-ACS Randomized Controlled Trial results were published end of July 2020 confirming the ...
Juan F. Granada, M.D., CEO of the Cardiovascular Research Foundation (CRF) worked on preclinical development work for a ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 9, 2020 — The Society of Interventional Radiology (SIR) published new clinical practice guidelines that ...
September 8, 2020 — Researchers developed a new class of medical instruments equipped with an advanced soft electronics ...
September 4, 2020 — The U.S. Centers for Medicare and Medicaid Services (CMS) granted a New Technology Add-on Payment ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

September 3, 2020 — Abbott today announced the start of the LIFE-BTK clinical trial to evaluate the safety and ...
September 2, 2020 — An international, first-of-its-kind cardiology trial used personalized genetic testing to reduce by ...
With COVID-19 forcing all medical conferences to go virtual in 2020, Juan F. Granada, M.D., CEO of the Cardiovascular ...


 September 16, 2020
September 16, 2020