February 4, 2021 — Transcatheter aortic valve replacement (TAVR) has historically only been used with caution in the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 3, 2021 — Modern Vascular is a medical group that has 13 outpatient cath lab clinics to treat peripheral artery ...
January, 27, 2021 — The U.S. Food and Drug Administration (FDA) has granted Occlutech a Breakthrough Device designation ...
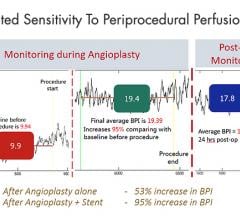
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 26, 2021 — Pedra Technology, a privately held company, announced today that the U.S. Food and Drug ...
January 26, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific's Synergy Megatron Drug ...

With increasing complexity of interventional structural heart disease and congenital heart disease interventions, 3-D ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 20, 2021 — The U.S. Centers for Medicare and Medicaid Services (CMS) revised its National Coverage Determination ...

January 19, 2021 – With the postponement of non-essential elective surgeries and medical procedures in 2020 to conserve ...
January 11, 2021 — Medtronic has received a new expanded indication from Health Canada for its Evolut Transcatheter ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 8, 2021 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Inari Medical Inc ...
January 6, 2021 — The U.S. Food and Drug Administration (FDA) has granted the designation of breakthrough device to P+F ...
December 29, 2020 — Edwards Lifesciences announced Dec. 21 that the first patient was treated in the RESTORE clinical ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
December 29, 2020 — Health Canada has approved the expanded use of the Edwards Lifesciences Sapien 3 and Sapien 3 Ultra ...

Here are the top 25 best performing articles on the Diagnostic and Interventional Cardiology (DAIC) website from 2020 ...
Here are the top performing 25 videos on the DAIC website in 2020. The picks are based on Google Analytics of the DAIC ...


 February 04, 2021
February 04, 2021