March 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 24, 2021 — A study of admissions for acute myocardial infarction (AMI) and heart failure (HF) in the U.K ...
February 24, 2021 — Occlutech announced the completion of patient enrollment in the PRELIEVE trial, pilot study to ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 24, 2021 – German-based endovascular simulator specialist Cathi has launched a highly portable System in a ...
February 23, 2021 — Medtronic has voluntarily issued a global recall of unused Medtronic Valiant Navion thoracic stent ...
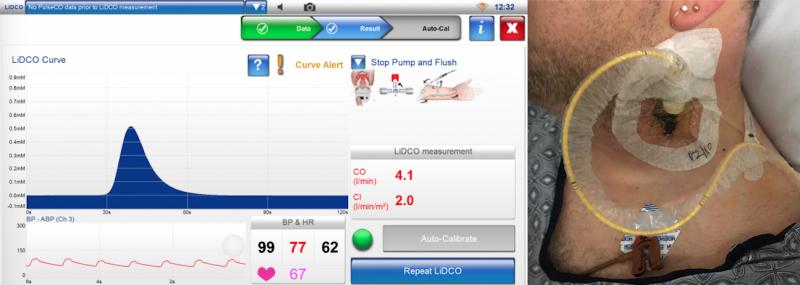
The overall trend in the cardiac output monitoring market is a movement toward noninvasive or minimally monitoring ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 22, 2021 — The U.S. Food and Drug Administration (FDA) recently cleared the Cook Medical Zilver Vena Venous ...

February 17, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Shockwave Medical's Intravascular ...
February 16, 2021 – SpectraWave Inc. announced a $13.2 million series A-2 financing to support completion of product ...
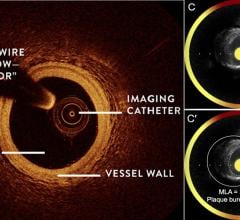
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 9, 2021 — InspireMD Inc., developer of the CGuard Embolic Prevention System (EPS) for the prevention of stroke ...
February 9, 2021 — EndoWays, developer of a disposable robotic system for the cath lab, announced the completion of an ...
February 8, 2021 — Teleflex Inc. said it recently completed its acquisition of Z-Medica LLC, an industry-leading ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Cindy Grines, M.D., MSCAI, FACC, president of the Society for Cardiovascular Angiography and Interventions (SCAI), and ...
February 4, 2021 — Transcatheter aortic valve replacement (TAVR) has historically only been used with caution in the ...
February 3, 2021 — Modern Vascular is a medical group that has 13 outpatient cath lab clinics to treat peripheral artery ...

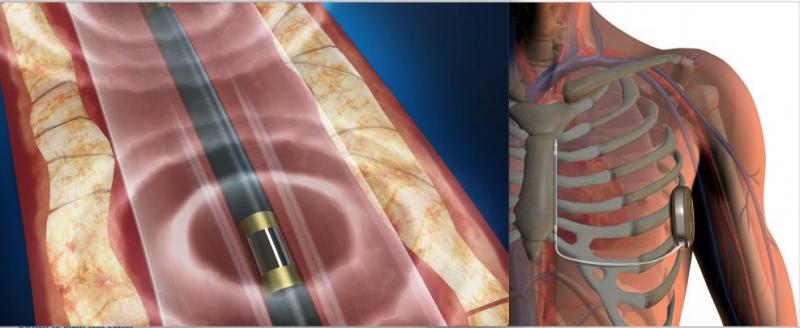
 March 01, 2021
March 01, 2021