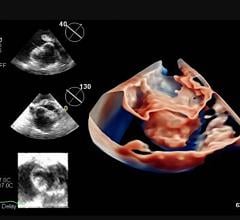
July 16, 2021 — Mayo Clinic recently became the first to use a next-generation 4-D intracardiac echo (ICE) imaging ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 15, 2021 — HeartFlow, which has commercialized noninvasive computed tomography derived fractional flow reserve (FFR ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Doctor Andreas Ruck, interventional cardiologist and head of the mitral/tricuspid program, Karolinska University ...
July 13, 2021 — East End Medical announced it received U.S. Food and Drug Administration (FDA) clearance for the company ...
July 7, 2021 – Robocath, a company that designs, develops and commercializes cath lab robotic solutions to treat ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 1, 2021 — The U.S. Food and Drug Administration (FDA) has cleared the Angel Medical Systems Inc. second-generation ...
June 30, 2021 — Abbott announced its Xience family of drug-eluting coronary stents received U.S. Food and Drug ...
June 29, 2021 – Cardiologists at Beaumont Health successfully replaced a 34-year-old woman’s tricuspid valve in a rare ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 29, 2021 - ScImage Inc.has partnered with National Cardiovascular Management (NCM), a national consulting and ...
June 28, 2021 — CentraCare, one of the largest health systems in Minnesota, successfully completed the first structural ...
June 24, 2021 — Data captured in American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) regis ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 22, 2021 – Philips Healthcare announced the official start of the DEFINE GPS study, with the first patient being ...
June 21, 2021 — The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim's dabigatran etexilate ...
It is brutal and very expensive to bring a new transcatheter valve to market. Boston Scientific invested vast amounts of ...

 July 16, 2021
July 16, 2021