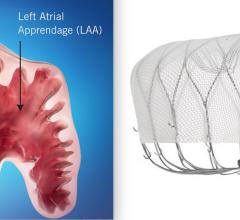
November 8, 2021 – SWISS-APERO is the first randomized clinical trial comparing the Abbott Amulet left atrial appendage ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
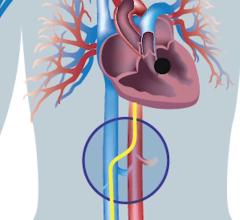
November 8, 2021 — Results from a clinical trial of the Edwards Lifesciences Evoque transcatheter tricuspid valve ...
November 5, 2021 – An FDA-approved device used during cardiac cath lab procedures cut radiation exposure for ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) ...
October 28, 2021 – The first patient has been treated in a new trial using stem cell therapy to treat chronic myocardial ...
October 27, 2021 — Teleflex Inc. announced the completion of patient enrollment in a clinical study evaluating the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

Intravascular ultrasound (IVUS) is the workhorse intravascular imaging modality in cardiac cath labs. It is used to help ...
October 20, 2021 — HeartFlow Inc. announced the commencement of the REVEALPLAQUE (A pRospEctiVe, multicEnter study to ...
October 20, 2021 — Philips, a global leader in health technology and intravascular ultrasound (IVUS) solutions ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 20, 2021 — 18-month results from the PRESTIGE* Below-the-Knee (BTK) study have been presented as a late-breaking ...
October 20, 2021 — Boston Scientific Corporation announced positive data for the Eluvia Drug-Eluting Vascular Stent ...
Dr. Neil Moat, MBBS, chief medical officer of Abbott's structural heart business, was a cardiac surgeon specializing in ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 15, 2021 — The American Heart Association (AHA) released a new policy statement that offers policy ...
October 15, 2021 — The InspireMD CGuard Embolic Prevention Stent System (EPS) device for the treatment of carotid artery ...
Tiberio Frisoli, M.D., interventional structural cardiologist, senior staff physician, Henry Ford Hospital, explains how ...

 November 08, 2021
November 08, 2021