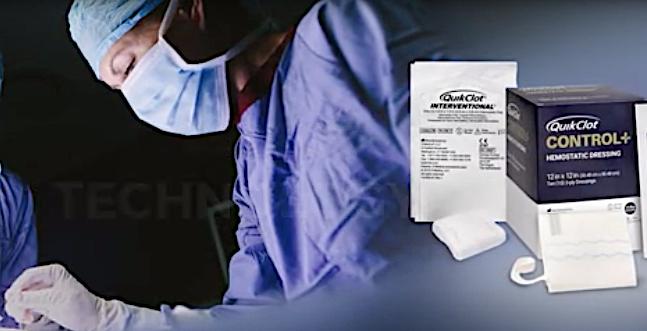
QuickClot uses a non-woven material impregnated with kaolin, an inorganic mineral. When kaolin contacts blood it activates Factor XII, which accelerates the body’s natural clotting cascade.
October 27, 2021 — Teleflex Inc. announced the completion of patient enrollment in a clinical study evaluating the performance of the QuikClot Control+ hemostatic device for mild to moderate (Class I and II) bleeding in cardiac procedures as compared to standard gauze.
QuickClot already has an FDA-cleared product for use for vascular access hemostasis management in cath lab procedures. The hemostatic technology consists of a non-woven material impregnated with kaolin, an inorganic mineral. When kaolin contacts blood it activates Factor XII, which accelerates the body’s natural clotting cascade.
The primary efficacy endpoint of this prospective, randomized, controlled Cardiac Investigational Device Exemption (IDE) Study is the rate at which subjects achieved hemostasis through 10 minutes of hemostatic application and compression at the bleeding site. The secondary efficacy endpoint is the proportion of subjects achieving hemostasis measured at 5 and 10 minutes. The study enrolled 231 patients across seven investigational sites in the U.S. and completed enrollment three months ahead of schedule.
The principal investigators of the Cardiac IDE study include Mubashir A. Mumtaz, M.D., chief of cardiothoracic surgery and surgical director of the structural heart program at UPMC Central Pennsylvania, and Marc Moon, M.D., section chief of cardiac surgery, at Washington University School of Medicine in St. Louis and past-president of the American Association for Thoracic Surgery (STS).
“We are pleased to participate in this study to explore the use of the QuikClot Control+ Hemostatic Device in cardiac surgery,” Mumtaz said. “We look forward to sharing the results of its use in various aspects of cardiac surgery including coronary artery bypass grafting (CABG), valve repair/replacement, and thoracic aortic surgeries.”
The QuikClot Control+ Hemostatic Device is the first FDA-cleared hemostatic dressing indicated for severe (Class III and IV) bleeding in the internal organ space and is currently commercially available in the U.S. The product is considered an investigational device for clinical evaluation in the Cardiac IDE study.
“More than 600,000 cardiothoracic procedures are performed in the U.S. each year, according to Teleflex research,” said Kevin Robinson, president and general manager, anesthesia and emergency medicine division, Teleflex. “Our goal is to offer surgeons another tool to support positive patient outcomes in these procedures. We anticipate submitting a 510(k) filing for expanded use of the QuikClot Control+ Device following completion of the study analysis.”
For more information: teleflex.com


 January 15, 2026
January 15, 2026