February 24, 2012 — 3-D Surgical Solutions LLC and Wheaton Franciscan-The Wisconsin Heart Hospital Campus announced that ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 24, 2012 — A new report released today by HealthGrades found that from 2008 to 2010, emergency admissions for ...
February 24, 2012 – The U.S. Food and Drug Administration (FDA) this week granted the first coronary stent indication ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 20, 2012 — ReCor Medical announced that its Paradise percutaneous ultrasound renal denervation system for ...
February 20, 2012 — Medtronic announced U.S. Food and Drug Administration (FDA) approval of the Resolute Integrity drug ...

As catheter-based, minimally invasive procedures expand rapidly beyond treatment of the coronary arteries into all areas ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 16, 2012 — Siemens Healthcare received clearance from the U.S. Food and Drug Administration (FDA) for syngo Aor ...
February 16, 2012 — Velomedix Inc., a clinical stage medical device company, received investigational device exemption ...
February 15, 2012 — Vessix Vascular Inc., developer of a novel percutaneous radiofrequency (RF) balloon catheter ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
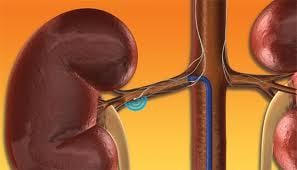
February 15, 2012 — Medtronic Inc. announced the start of two clinical initiatives evaluating the broader, real-world ...
February 10, 2012—Merit Medical Systems recently announced it acquired the assets of Ostial Solutions LLC, maker of the ...
February 10, 2012 — The U.S. Food and Drug Administration (FDA) sent a warning letter to Merit Medical Systems Inc ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
February 9, 2012 — Stereotaxis Inc. said its Vdrive robotic navigation system, which provides physicians the ability to ...
February 9, 2012 — Boston Scientific announced the United States launch of the TruePath CTO device, designed to ...
February 9, 2012 — DSM announced a successful collaboration with medical manufacturer EPflex Feinwerktechnik GmbH to ...


 February 24, 2012
February 24, 2012












