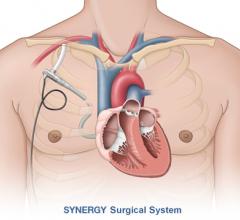
May 24, 2013 — Although not specifically designed for cardiovascular surgery, a new robotic surgical system being ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
May 21, 2013 — Zoll Medical Corp. has received Shonin approval from the Japanese Ministry of Health, Labour and Welfare ...
May 20, 2013 — If someone suffers a heart attack while walking down the street and is taken to the hospital quickly, his ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
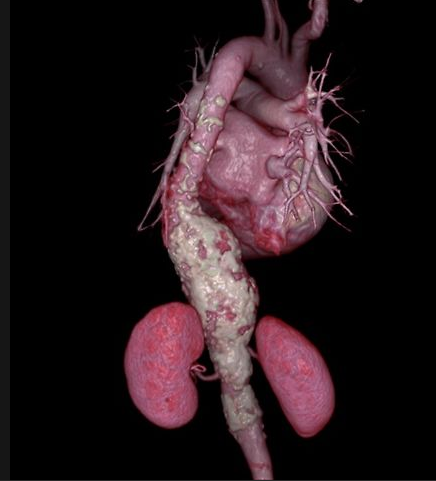
May 20, 2013 — Patients with ruptured abdominal aortic aneurysms (AAA) are more than twice as likely to survive if they ...
May 20, 2013 — CircuLite Inc. announced that it has received approval from the Federal Agency for Medicines and Health ...
May 17, 2013 — Can high blood pressure be safely reduced and controlled by “disconnecting” nerves in the kidneys? That ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 17, 2013 — Patients with coronary artery disease who undergo treatment at the University of Maryland Medical Center ...

May 17, 2013 — Prof. Dr. Béla Merkely and Dr. Péter Sótonyi at Semmelweis Egyetem Kardiológiai Központ in Hungary ...

May 17, 2013 — The Journal of American College of Cardiology has published the results from Corindus Vascular Robotics’ ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

May 16, 2013 — St. Jude Medical gained CE mark approval of its Ilumien Optis percutaneous coronary intervention (PCI) ...

May 16, 2013 — Elixir Medical Corp. announced it received CE (Conformité Européenne) mark approval for its DESolve ...

May 15, 2013 — In 2012, more than 3 million people had stents inserted in their coronary arteries. After about six ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 15, 2013 — Vascular Solutions Inc. announced that it has re-launched the Venture catheter, a deflectable-tip cathete ...
May 14, 2013 — Toshiba America Medical Systems Inc. offers electrophysiology (EP) clinicians an Infinix-i cardiovascular ...

The development of 3-D transesophageal echo (TEE) just a few years ago has enabled a new generation of interventional ...


 May 24, 2013
May 24, 2013