July 22, 2013 — Toshiba America Medical Systems Inc. has partnered with Unfors RaySafe Inc. to offer a new radiation ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 22, 2013 — A new report issued by the American College of Cardiology (ACC) and developed in collaboration with 10 ...
July 18, 2013 — Abbott announced that it has entered into an agreement to purchase IDEV Technologies, a privately held ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
July 18, 2013 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) 510(k) clearance and CE ...
July 15, 2013 — Janssen Research & Development LLC announced the U.S. Food and Drug Administration (FDA) has issued a ...

July 12, 2013 — Virginia Anderer, a Chicago native living in southern California, was the first patient in San Diego and ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 12, 2013 — Micell Technologies Inc. received CE mark approval for its MiStent sirolimus-eluting absorbable polymer ...

July 11, 2013 — In a clinical study that included nearly 6 million Medicare Advantage and Medicare fee-for-service ...
July 9, 2013 — Researchers have announced the results of a clinical study that shows a key difference in the patient’s ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 8, 2013 — Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to ...
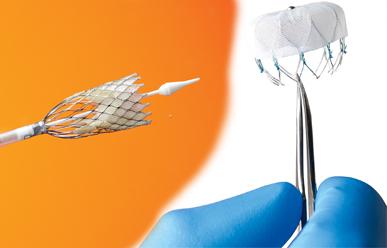
The field of interventional cardiology is one of constant change and innovation. The past 10 years have documented ...

The year 2013 has brought us several important clinical trials that have changed the way interventional cardiologists ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 1, 2013 — One-year data from an Italian multicenter randomized controlled trial of the In.Pact Falcon drug-eluting ...
July 1, 2013 — Primary end-point results from the BIOFLOW-II clinical study demonstrating the non-inferiority of the ...
June 25, 2013 — Cordis Corp. announced the European CE marking and U.S. Food and Drug Administration (FDA) approval of ...


 July 22, 2013
July 22, 2013






