September 17, 2013 — In a significant milestone toward obtaining additional key regulatory approvals for the Synergy dru ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
There is no shortage of debate among interventional cardiologists over whether vascular closure devices should be used ...

Vascular closure devices (VCD) were first introduced in the early 1990s with a goal to achieve quick hemostasis, thus ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
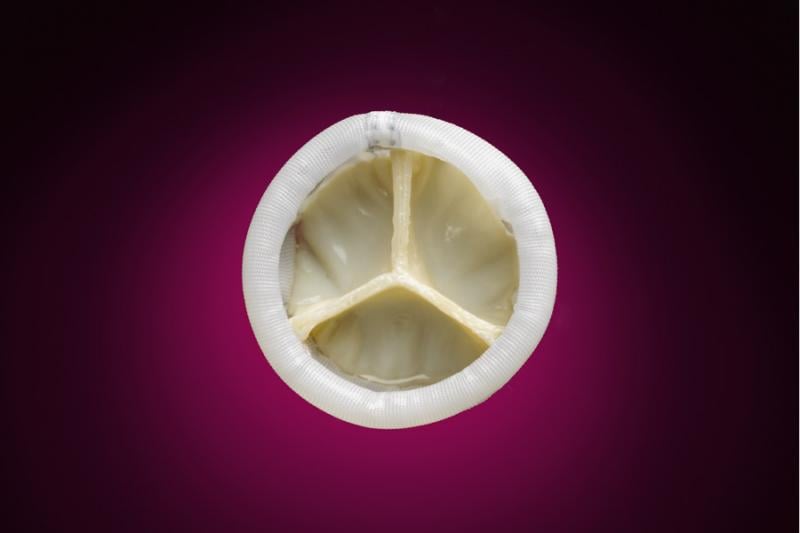
September 11, 2013 — Direct Flow Medical Inc. announced the first patient enrollment in the U.S. SALUS clinical trial ...
September 9, 2013 — Keystone Heart, a company in the development of cerebral protection devices for interventional ...
September 9, 2013 — Cardiac stents to open blocked heart arteries and reduce chest pain have been used for decades ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 9, 2013 — Medtronic Inc. has formed Medtronic Hospital Solutions, a new business focused on developing novel ...
September 5, 2013 — Use of the novel anticoagulant otamixaban did not reduce ischemic events compared with ...
September 4, 2013 — Terumo has introduced the GlideSheath Slender Hydrophilic Introducer Sheath, a 6 French sheath that ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

September 3, 2013 — St. Jude Medical Inc. announced the CE mark approval of its next-generation EnligHTN renal ...
September 3, 2013 — Avinger Inc. has received CE Mark approval for Pantheris – a system that combines directional athere ...
August 30, 2013 — PFM Medical received U.S. Food and Drug Administration (FDA) premarket approval (PMA) for its Nit ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
August 29, 2013 — Bayer HealthCare announced that the U.S. Food and Drug Administration (FDA) has cleared two new ...
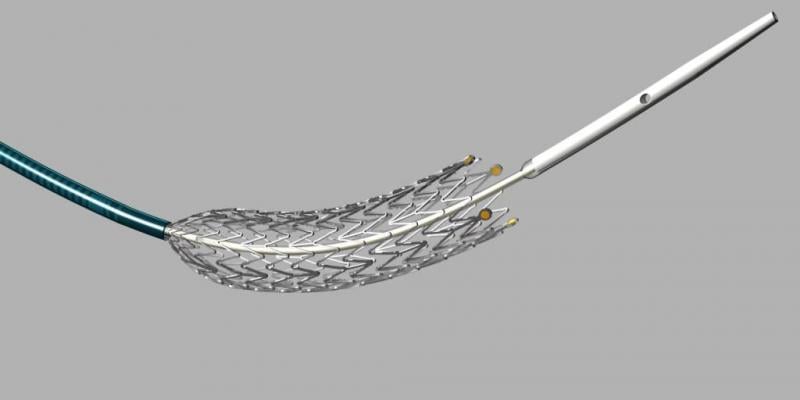
August 28, 2013 — Effective Oct. 1, Cook Medical’s Zilver PTX drug-eluting peripheral stent qualifies for new-technology ...
|
August 28, 2013 — According to Millennium Research Group (MRG), efforts to streamline the regulatory approval process in ... |


 September 17, 2013
September 17, 2013