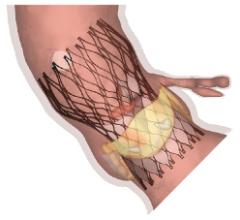
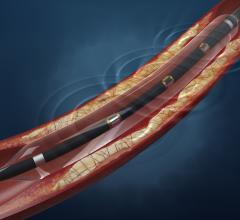
February 2, 2015 — Biosensors International Group has announced a distribution agreement with Veryan Medical Ltd. for Bi ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
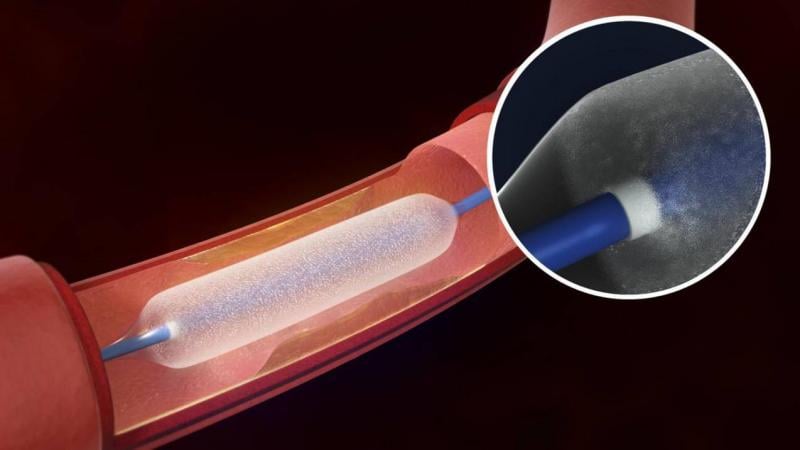
January 30, 2015 — ECRI Institute has created a report that offers an overview of drug-eluting balloon (DEB) technology ...
January 29, 2015 — Merge Healthcare Inc. announced that Merge Cardio has been named Best in KLAS for the cardiology ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

There are several new interventional and minimally invasive surgical heart failure (HF) devices in development or in ...
January 28, 2015 — Neovasc Inc. has commenced a public offering in the United States of 8 million shares of the company ...
January 28, 2015 — Maquet Medical Systems USA announced an agreement to serve as the exclusive U.S. distributor of ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 26, 2015 — FEops announced the closing of a €1.3 million (~$1.9 million USD) series A financing round, led by ...
January 22, 2015 — AstraZeneca announced that the PEGASUS-TIMI 54 study, a large-scale outcomes trial involving more ...
The America College of Cardiology has released its list of key late-breaking clinical trials at the ACC 2015 meeting in ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 21, 2015 — When retrievable inferior vena cava (IVC) filters were approved for use in the United States in 2003 ...
January 16, 2015 — Bellerophon Therapeutics LLC said it completed enrollment of its PRESERVATION I clinical trial of ...
January 15, 2015 — The Society of Interventional Radiology (SIR) unveiled a new brand for the society and its research ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 14, 2015 — Opsens Inc. announced the first use of its Fractional Flow Reserve (FFR) products in Europe by the ...
January 13, 2015 — Shockwave Medical announced CE Mark regulatory approval for the company’s Lithoplasty balloon ...
January 9, 2015 — Covidien announced it has received CE Mark approval for its Stellarex drug-coated angioplasty balloon ...


 February 02, 2015
February 02, 2015