October 26, 2016 — Philips recently announced its latest image guidance solutions to be featured at the 2016 ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
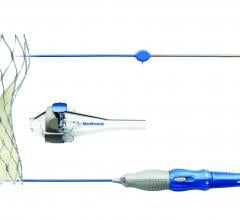
October 26, 2016 — Medtronic plc announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the ...
(Editor’s note: This is the second part of a two-part series on the proposed Medicare five-year demonstration for a ...
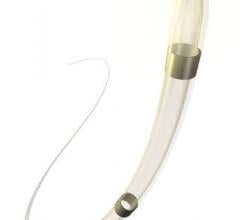
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
October 24, 2016 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the HawkOne ...
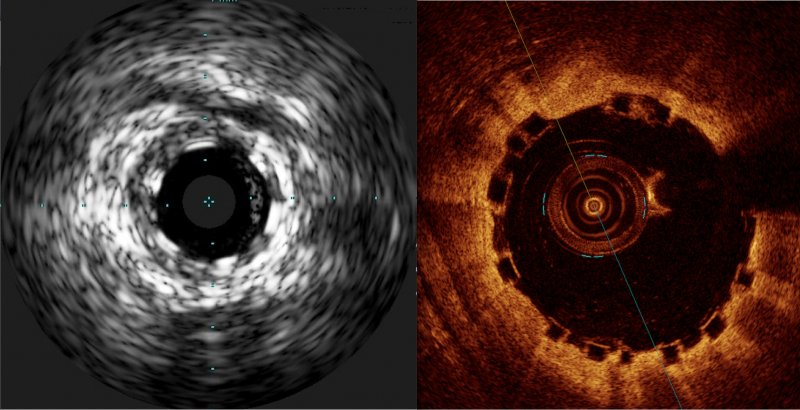
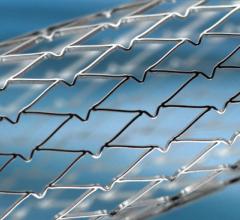
There has been a lot of interest in the interventional community regarding the Abbott Absorb Bioresorbable Vascular ...
October 20, 2016 — Avinger Inc. recently announced that the company has received expanded indications from the U.S. Food ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 19, 2016 — St. Jude Medical Inc. announced the launch of the ADO II AS (AMPLATZER Duct Occluder II Additional ...
October 18, 2016 — Medtronic plc announced last week that it has notified customers of a voluntary recall of certain ...
As healthcare moves into the era of bundled payments, providers need to be especially focused on ensuring delivery of ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 18, 2016 — Abbott and St. Jude Medical Inc. announced today an agreement to sell certain products from their ...
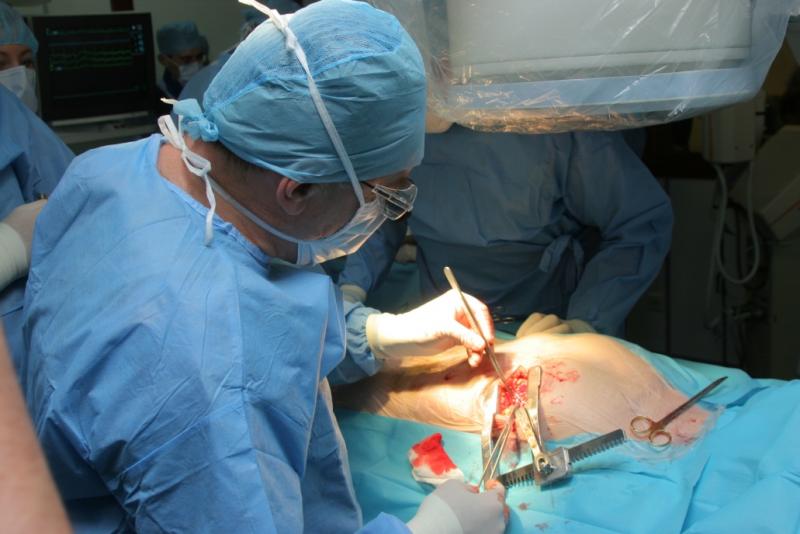
October 13, 2016 — A surveillance project to evaluate the safety and effectiveness of transcarotid artery ...
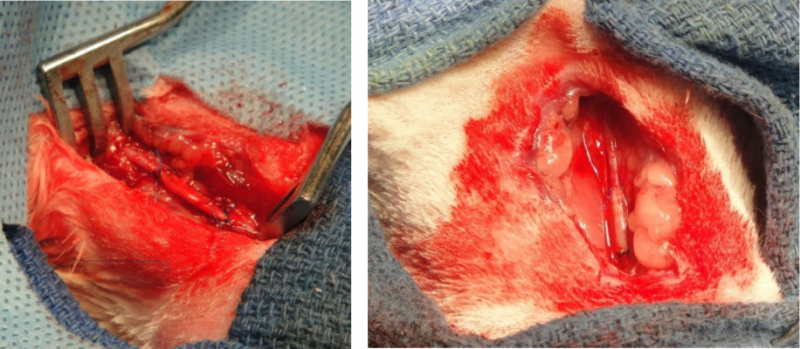
The U.S. Food and Drug Administration (FDA) changed its rules concerning custom medical devices Oct. 12, adding new ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 12, 2016 — VIVA (Vascular Interventional Advances) Physicians announced a number of highly anticipated late ...

While guidewires are a key tool used by all interventionalists in the cath lab, most operators do not have a deep ...
October 12, 2016 — Medtronic plc announced recently that the U.S. Food and Drug Administration (FDA) has cleared the ...


 October 26, 2016
October 26, 2016