March 19, 2009 - The ZOLL Medical Corp. LifeVest Wearable Defibrillator has been prescribed for patients by ...
Cardiac Diagnostics
This channel includes news, videos, podcasts and other content on new technology innovations for cardiac diagnostic systems and techniques. This includes laboratory testing, blood tests including troponin testing, electrocardiogram (ECG) systems, point of care testing systems, genetic testing, cardiac patient monitoring devices including wearable sensors, and studies showing new ways to diagnose heart diseases.
March 20, 2009 - BRAHMS AG of Hennigsdorf, Germany and Annapolis, MD, recently submitted a 510(k) file to the FDA ...
March 19, 2009 - Sorin Group and Orange Business Services today entered into an agreement to develop and service a ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 18, 2009 - GE said yesterday it was awarded two 10-year contracts by the Defense Supply Center Philadelphia ...
March 18, 2009 - Vicor Technologies Inc. has entered into a collaborative research agreement with the University ...
March 17, 2009 - eCardio Diagnostics has launched an Academic Medicine initiative to provide a tailored offering ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
March 16, 2009 - St. Jude Medical Inc. today announced FDA clearance of the newest version of the Merlin.net ...
Patients with chronic conditions such as congestive heart failure use Honeywell HomMed’s Genesis DM telehealth ...
March 13, 2009 - Medical testing meets medical texting with the FDA’s clearance of GE Healthcare’s latest ECG ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
March 11, 2009 - The VAP Cholesterol Test from Atherotech Inc. is now available with apolipoprotein A (apoAI) ...
March 11, 2009 – The use of common screening tests, such as the ankle brachial index (ABI), along with ...
Door-to-balloon time can be used as a quality measure for hospitals in their level of excellence in caring for ...
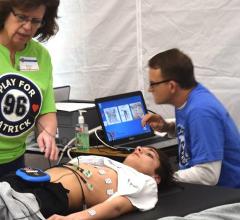
Sudden cardiac death (SCD) is the leading medical cause of death in young athletes and its impact is consistent worldwide. Most professional athletes in the United States are required to take part in comprehensive cardiovascular screening programs to identify often-asymptomatic congenital or inherited heart disorders, and other cardiac risk factors. There remains a debate however, whether to mandate ECGs as part of pre-participation screening programs for student athletes at the collegiate and high school levels or even at younger ages.

With the healthcare industry striving for greater efficiency at virtually every level, it shouldn’t be a surprise ...
March 9, 2009 - The Ministry of Health for the Province of Quebec has awarded ZOLL Medical Corp. a contract valued ...
March 6, 2009 – VectraCor Inc. submitted its 510(k) application to the FDA in December for a new technology to ...


 March 20, 2009
March 20, 2009