December 14, 2011 — The results of the COMPASS (Coronary Obstruction Detection by Molecular Personalized Gene Expression ...
Cardiac Diagnostics
This channel includes news, videos, podcasts and other content on new technology innovations for cardiac diagnostic systems and techniques. This includes laboratory testing, blood tests including troponin testing, electrocardiogram (ECG) systems, point of care testing systems, genetic testing, cardiac patient monitoring devices including wearable sensors, and studies showing new ways to diagnose heart diseases.
December 12, 2011 - CardioMEMS announced that the U.S. Food and Drug Administration's (FDA) Circulatory Systems Devices ...
December 6, 2011 — Kaleida Health, part of the Buffalo Heart Group in Buffalo, N.Y., announced its first implant of the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
December 5, 2011 – Roche announced that it has signed an agreement under which it will acquire 100 percent of Verum ...
November 18, 2011 – The first comprehensive report to review timing of treatment for patients with blockages in more ...
November 18, 2011 – Medtronic and Bain Capital, a global private investment firm, today announced they have entered into ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
November 16, 2011 — The American Society of Echocardiography (ASE) issued a response voicing concerns over the ...
November 16, 2011 – Biological Signal Processing (BSP), which develops and manufactures products for the non-invasive ...
November 15, 2011 — A clinical trial of patients undergoing percutaneous coronary intervention (PCI) for acute coronary ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
November 14, 2011 — Everist Genomics announced plans to commercialize its CardioDefender diagnostic system. It is the ...
November 14, 2011 – ProTron Technologies announces a new approach to early detection of aneurysms in vascular systems ...
November 4, 2011 – PhysioSonics announced that it received a $2.5 million grant from the Telemedicine and Advanced ...
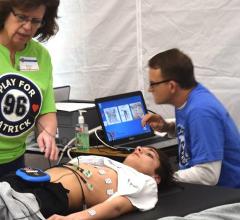
Sudden cardiac death (SCD) is the leading medical cause of death in young athletes and its impact is consistent worldwide. Most professional athletes in the United States are required to take part in comprehensive cardiovascular screening programs to identify often-asymptomatic congenital or inherited heart disorders, and other cardiac risk factors. There remains a debate however, whether to mandate ECGs as part of pre-participation screening programs for student athletes at the collegiate and high school levels or even at younger ages.
November 3, 2011 – New study indicates strong diagnostic ability of treadmill exercise stress testing to uncover Long QT ...
November 1, 2011 – CardioComm Solutions, a provider of hardware and software solutions for cardiovascular health ...
October 31, 2011 — On Oct. 18, 2011, the TICH-BIOTRONIC Pacemaker Wireless Monitoring Services Centre was co-founded in ...


 December 14, 2011
December 14, 2011