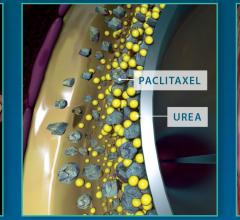
June 28, 2017 — Philips Healthcare and Spectranetics Corp. announced they have entered into a definitive merger ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.

June 12, 2017 – Med Alliance announced completion on schedule of patient enrollment in the first-in-man (FIM) study of ...
May 22, 2017 — Abbott Vascular has initiated a voluntary recall of specific lots of three catheters due to 19 reports of ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
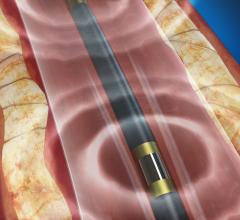
This video, provided by Spectranetics, demonstrates how to deploy the Bridge Occlusion Balloon used to seal accidental ...
May 17, 2017 — Contego Medical LLC announced recently that it has received CE Marking of its Vanguard IEP Peripheral ...
April 26, 2017 — Boston Scientific announced results from the RANGER SFA trial for the Ranger Paclitaxel-Coated PTA Ball ...
March 9, 2017 — Medtronic plc announced the launch of the IN.PACT BTK study to evaluate the effectiveness of a drug ...
February 8, 2017 — Medtronic plc announced receipt of an investigational device exemption (IDE) from the U.S. Food and ...
December 6, 2016 — The Spectranetics Corp. announced in late November that its Stellarex 0.014-inch drug-coated ...
A discussion with Todd Brinton, M.D., about the newly FDA-cleared Shockwave Medical Lithoplasty System, at the ...
September 26, 2016 — C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational ...
September 22, 2016 — New data presented at the Vascular Interventional Advances (VIVA) conference demonstrated the ...
September 19, 2016 — Medtronic plc announced that the U.S. Food and Drug Administration (FDA) approved the IN.PACT ...
September 16, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance for Shockwave Medical’s ...
This video, provided by Shockwave Medical, demonstrates the Lithoplasty System. It uses ultrasonic waves to treatment ...

 June 28, 2017
June 28, 2017