May 30, 2014 — New research has found that bariatric surgery is an effective way to control weight in morbidly obese ...
Atrial Fibrillation
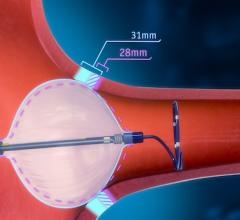
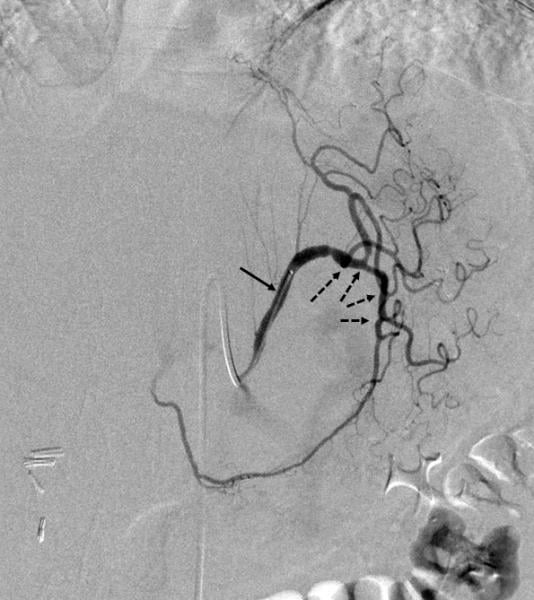
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
May 30, 2014 — Alere announced a class I recall of its INRatio2 PT/INR professional test strips, part of the Alere ...
May 16, 2014 — New data presented as a late-breaking clinical trial at the Heart Rhythm Society's (HRS) 2014 annual ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 12, 2014 — Kalila Medical received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Vado ...

The American College of Cardiology (ACC) annual meeting is a key event where vendors launch new cardiology technologies ...

April 10, 2014 — The 2014 Guideline for the Management of Patients With Atrial Fibrillation includes recommendations for ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...

February 28, 2014 — The U.S. Food and Drug Administration (FDA) approved Biosense Webster Inc.’s Thermocool Smarttouch ...
February 27, 2014 — Approximately one in two patients with atrial fibrillation (Afib) do not optimally reduce their risk ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
February 27, 2014 — Apama Medical is a privately held medical device company formed by Shifamed's medical incubator. It ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

 May 30, 2014
May 30, 2014