July 7, 2016 — AtriCure Inc. announced the first patient has been enrolled in the FROST study at William Beaumont ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
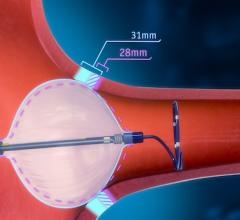
July 5, 2016 — AtriCure Inc. announced that it has received CE Mark for the AtriClip PRO2 Left Atrial Appendage (LAA) ...
June 28, 2016 — New research shows patients admitted to National Health Service (NHS) hospitals in the United Kingdom ...
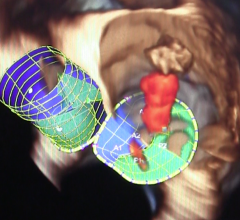
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
June 24, 2016 — More than 1 in 3 atrial fibrillation (AF) patients at intermediate to high risk for stroke are treated ...
June 2, 2016 — Female atrial fibrillation patients are less likely than their male counterparts to receive blood ...
May 25, 2016 — Technavio’s latest report on the U.S. anticoagulants market provides an analysis on the most important ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...

Atrial fibrillation (AF) affects nearly 6 million Americans and the condition puts them at significantly greater risk of ...
May 19, 2016 — Physician-researchers in the College of Medicine at the University of Cincinnati have developed a ...
May 19, 2016 — St. Jude Medical Inc. announced results from two cardiovascular clinical trials presented at EuroPCR 2016 ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
May 16, 2016 — Tricuspid regurgitation (TR) occurs when the heart’s tricuspid valve leaks, allowing blood to flow back ...
May 11, 2016 — Patients with Wolff-Parkinson-White syndrome who receive catheter ablation to cure their abnormal heart ...
May 9, 2016 — Results from a first-of-its-kind study identify a significant increase in the incidence of atrial ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 9, 2016 — A new study shows the use of novel anticoagulants to treat atrial fibrillation (AF) on an “as needed basis ...
May 9, 2016 — A new study has found an increased risk of dementia in patients with atrial fibrillation (AF) that receive ...
May 9, 2016 — A new study presented at the Heart Rhythm Society’s 37th Annual Scientific Sessions, found that among ...


 July 07, 2016
July 07, 2016