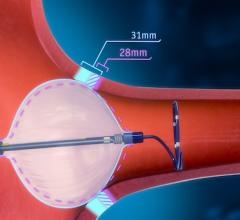
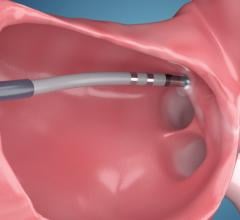
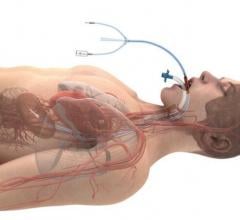
May 15, 2021 — Patients with an elevated risk of stroke due to heart rhythm problems, or atrial fibrillation (AFib) ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
May 12, 2021 — Galaxy Medical and Japan Lifeline announced an exclusive distribution agreement for the Alpha1 ablation ...
April 19, 2021 — National Heart, Lung, and Blood Institute (NHLBI) has awarded a grant for $462,689 to Rhythm ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
March 23, 2021 — Researchers at Columbia University are using cardiac ultrasound to improve the critical need to ...

The latest cardiology practice-changing scientific breakthrough, late-breaking study presentations have been announced ...
March 3, 2021 — Farapulse Inc. announced the first patients were treated in the ADVENT Trial, a U.S. Food and Drug ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
March 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
January 29, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Medtronic's DiamondTemp Ablation (DTA) system ...
In our most challenging and distressing days, it is nice to think about a post-COVID-19 world.
But while this ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
December 23, 2020 — The American College of Cardiology (ACC) and the American Heart Association (AHA) has made two ...
Oussama Wazni, M.D., section head, electrophysiology, Cleveland Clinic, discusses the results of the recent STOP AF ...
December 3, 2020 – Attune Medical reports the first major peer-reviewed clinical publication using its ensoETM device in ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
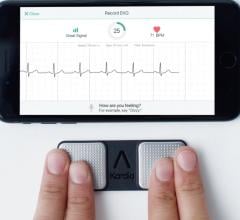
November 24, 2020 — The U.S. Food and Drug Administration (FDA) has cleared AliveCor's next generation of interpretive a ...
Steven Lubitz, M.D., MPH, cardiac electrophysiologist, Massachusetts General Hospital, presented the late-breaking VITAL ...
November 16, 2020 — The three-year clinical outcomes of the mHealth Screening to Prevent Strokes (mSToPS) study ...

 May 15, 2021
May 15, 2021